Cuprate

Cuprate loosely refers to a material that can be viewed as containing copper anions. Examples include tetrachlorocuprate ([CuCl4]2-), the superconductor YBa2Cu3O7, and the organocuprates ([Cu(CH3)2]-).[2] The term cuprates derives from the Latin word for copper, cuprum. The term is mainly used in three contexts - oxide materials, anionic coordination complexes, and anionic organocopper compounds.
Oxides
One of the simplest oxide-based cuprates is the copper(III) oxide KCuO2, a dark blue diamagnetic solid. It is produced by heating potassium peroxide and copper(II) oxide in an atmosphere of oxygen:[3]
- K2O2 + 2 CuO → 2 KCuO2
Interest in cuprates sharply increased in 1986 with the discovery of high-temperature superconductivity in the Non-stoichiometric cuprate lanthanum barium copper oxide La2-xBaxCuO4. The Tc for this material was 35 K, well above the previous record of 23K.[4] Thousands of publications examine the superconductivity in cuprates between 1986 and 2001,[5] and Bednorz and Müller were awarded the Nobel Prize in Physics only a year after their discovery.[6]
From 1986 to 2008, many cuprate superconductors were identified. Most famous is yttrium barium copper oxide (YBa2Cu3O7, "YBCO" or "1-2-3"). Another example is bismuth strontium calcium copper oxide (BSCCO or Bi2Sr2CanCun+1O2n+6-d) with Tc = 95–107 K depending on the n value. Thallium barium calcium copper oxide (TBCCO, TlmBa2Can−1CunO2n+m+2+δ) was the next class of high-Tc cuprate superconductors with Tc = 127 K observed in Tl2Ba2Ca2Cu3O10 (TBCCO-2223) in 1988.[7] The highest confirmed, ambient-pressure, Tc is 135 K, achieved in 1993 with the layered cuprate HgBa2Ca2Cu3O8+x.[8][9] Few months later, another team measured superconductivity above 150K in the same compound under applied pressure (153 K at 150 kbar).[10]
Cuprate superconductors usually feature copper oxides in both the oxidation state 3+ as well as 2+. For example, YBa2Cu3O7 is described as Y3+(Ba2+)2(Cu3+)(Cu2+)2(O2-)7. All superconducting cuprates are layered materials having a complex structure described as a superlattice of superconducting CuO2 layers separated by spacer layers where the misfit strain between different layers and dopants in the spacers induce a complex heterogeneity that in the superstripes scenario is intrinsic for high temperature superconductivity.
Applications
BSCCO superconductors already have large-scale applications. For example, tens of kilometers of BSCCO-2223 electrical cables are being used in the Large Hadron Collider – the world's largest and highest-energy particle accelerator.[11]
Coordination complexes
Copper forms many anionic "cuprate" coordination complexes with negatively charged ligands such as cyanide, hydroxide, and halides. Copper(I) derivatives tend to be colorless, copper(II) complexes are often turquoise-blue, and copper(III) and copper(IV) complexes are often orange-red.[12]
.png)
One example of a copper(I)-based cuprate is tetracyanocuprate(I), [Cu(CN)4]3–.
Copper(II) anions are most common, especially the chlorocuprates, such as trichlorocuprate(II) [CuCl3]–, tetrachlorocuprate(II) [CuCl4]2– and pentachlorocuprate(II) [CuCl5]3-.[2] The light blue solid sodium tetrahydroxycuprate is well known, it is prepared by heating cupric hydroxide with concentrated sodium hydroxide.[13]
- Cu(OH)2 + 2 NaOH → Na2Cu(OH)4
Dilithium tetrachlorocuprate (Li2CuCl4) is an effective catalyst for the couplings of alkyl halides in the Grignard reaction. It is prepared by mixing lithium chloride (LiCl) and copper(II) chloride (CuCl2) in tetrahydrofuran.[14]
There are also rare copper(III) and copper(IV) complexes such as the hexafluorocuprate(III) [CuF6]3– and hexafluorocuprate(IV) [CuF6]2–, which are strong oxidizing agents.
Organic cuprates
.png)
Cuprates play an important role in organic chemistry. The first organocopper compound, the explosive copper(I) acetylide Cu2C2, was synthesized by Rudolf Christian Böttger in 1859.[15]
Organic cuprates usually contain an R2Cu moiety, where R is a carbon containing unit. They are rather reactive towards oxygen and water forming copper(I) oxide; they are generally insoluble in inert solvents, tend to be thermally unstable and are difficult to handle. Nevertheless, organocopper reagents are frequently used in organic chemistry as alkylating reagents prepared in situ in an inert environment.[16] The electronegativity of copper is much higher than its neighbor in the group 12 elements, zinc, suggesting less nucleophilicity for carbon.
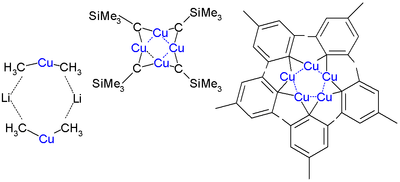 Organocopper aggregates containing more than one copper atom |
 Skeletal formula of a dimer from the crystal structure of lithium diphenylcuprate etherate, Ph2CuLi·2OEt2[1] |
 |
See also
- Benedict's reagent
- Rachel-House synthesis
- Category:copper compounds
- High-temperature superconductivity
| |||||||||||||||||
References
- ↑ Lorenzen, Nis Peter (1990). "Synthesis and Structure of a Dimeric Lithium Diphenylcuprate:[{Li(OEt)2}(CuPh2)]2". Angewandte Chemie International Edition in English 29: 300. doi:10.1002/anie.199003001.
- ↑ 2.0 2.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- ↑ "Potassium Cuprate (III)" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1015.
- ↑ J.G. Bednorz and K.A. Mueller (1986). "Possible high TC superconductivity in the Ba-La-Cu-O system". Z. Phys. B 64 (2): 189–193. Bibcode:1986ZPhyB..64..189B. doi:10.1007/BF01303701.
- ↑ Mark Buchanan (2001). "Mind the pseudogap". Nature 409 (6816): 8–11. doi:10.1038/35051238. PMID 11343081.
- ↑ Nobel prize autobiography
- ↑ Sheng, Z. Z.; Hermann A. M. (1988). "Bulk superconductivity at 120 K in the Tl–Ca/Ba–Cu–O system". Nature 332 (6160): 138–139. Bibcode:1988Natur.332..138S. doi:10.1038/332138a0.
- ↑ Schilling, A.; Cantoni, M.; Guo, J. D.; Ott, H. R. (1993). "Superconductivity above 130 K in the Hg–Ba–Ca–Cu–O system". Nature 363: 56–58. Bibcode:1993Natur.363...56S. doi:10.1038/363056a0.
- ↑ Lee, Patrick A (2008). "From high temperature superconductivity to quantum spin liquid: progress in strong correlation physics". Reports on Progress in Physics 71: 012501. arXiv:0708.2115. Bibcode:2008RPPh...71a2501L. doi:10.1088/0034-4885/71/1/012501.
- ↑ Chu, C. W.; Gao, L.; Chen, F.; Huang, Z. J.; Meng, R. L.; Xue, Y. Y. (1993). "Superconductivity above 150 K in HgBa2Ca2Cu3O8+δ at high pressures". Nature 365 (6444): 323. Bibcode:1993Natur.365..323C. doi:10.1038/365323a0.
- ↑ "HTS materials for LHC current leads". CERN.
- ↑ Egon Wiberg, Nils Wiberg, Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. pp. 1252–1264. ISBN 0-12-352651-5.
- ↑ "Sodium Tetrahydroxocuprate (II) in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1015.
- ↑ Atta-ur-Rahman (2002). Bioactive natural products. Elsevier. pp. 73;81;83. ISBN 0-444-51004-4.
- ↑ R. C. Böttger (1859). "Ueber die Einwirkung des Leuchtgases auf verschiedene Salzsolutionen, insbesondere auf eine ammoniakalische Kupferchlorürlösung". Annalen 109 (3): 351. doi:10.1002/jlac.18591090318.
- ↑ Louis S. Hegedus (1999). Transition metals in the synthesis of complex organic molecules. University Science Books. pp. 61–65. ISBN 1-891389-04-1.