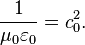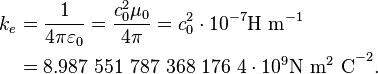Coulomb's constant
Coulomb's constant, the electric force constant, or the electrostatic constant (denoted ke ) is a proportionality constant in equations relating electric variables and is exactly equal to ke = 8.9875517873681764×109 N·m2/C2 (m/F). It was named after the French physicist Charles-Augustin de Coulomb (1736–1806).
Value of the constant
The exact value of Coulomb's constant ke comes from three of the fundamental, invariant quantities that define free space in the SI system: the speed of light c0 , magnetic permeability μ0 , and electric permittivity ε0 , related by Maxwell as:
Because of the way the SI base unit system made the natural units for electromagnetism, the speed of light in vacuum c0 is 299,792,458 m s−1, the magnetic permeability μ0 of free space is 4π·10−7 H m−1, and the electric permittivity ε0 of free space is 1 ⁄ (μ0 c2
0 ) ≈ 8.85418782×10−12 F m−1,[1]
so that[2]
Use of Coulomb's constant
Coulomb's constant is used in many electric equations, although it is sometimes expressed as the following product of the Vacuum permittivity constant:
 .
.
Some examples of use of Coulomb's constant are the following:
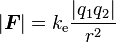 .
.
 .
.
 .
.
See also
References
- ↑ CODATA Value: electric constant. Physics.nist.gov. Retrieved on 2010-09-28.
- ↑ Coulomb's constant, Hyperphysics