Contryphan
| Contryphan | |||||||||
|---|---|---|---|---|---|---|---|---|---|
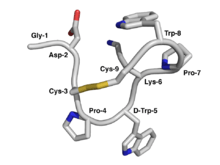 NMR structure of Contryphan-Vn. The peptide backbone is depicted by a curved tube while the amino acid side-chains are represented by capped sticks. Carbon atoms are colored grey, nitrogen atoms blue, oxygen atoms red, and sulfur atoms yellow.[1] . | |||||||||
| Identifiers | |||||||||
| Symbol | Contryphan_CS | ||||||||
| Pfam | PF02950 | ||||||||
| InterPro | IPR011062 | ||||||||
| PROSITE | PS60027 | ||||||||
| SCOP | 2cco | ||||||||
| SUPERFAMILY | 2cco | ||||||||
| |||||||||
The contryphans (conus + tryptophan) are a family of peptides that are active constituents of the poisonous venom produced by cone snail (genus conus). The two amino acid cysteine residues in contryphans are linked by a disulfide bond. In addition, contryphans undergo an unusual degree of post-translational modification including epimerization of leucine and tryptophan, tryptophan bromination, amidation of the C-terminus, and proline hydroxylation.[2]
Family members

Contryphan family members include:
| Peptide | Sequence | Species | Reference |
|---|---|---|---|
| Des(Gly1)contryphan-R | COwEPWC-NH2 | C. radiatus | [3] |
| Contryphan-R | GCOwEPWC-NH2 | C. radiatus | [3] |
| Bromocontyphan-R | GCOwEPXC-NH2 | C. radiatus | [4] |
| Contryphan-Sm | GCOwQPWC-NH2 | C. stercusmuscarum | [5] |
| Contryphan-P | GCOwDPWC-NH2 | C. purpurascens | [5] |
| Contryphan-R/Tx | GCOwEPWC-NH2 | C. textile | [5] |
| Contryphan-Tx | GCOWQPYC-NH2 | C. textile | [5] |
| Contryphan-Vn | GDCPwKPWC-NH2 | C. ventricosus | [6] |
| Leu-contryphan-P | GCVlLPWC-OH | C. purpurascens | [7] |
| Leu-contryphan-Tx | CVlYPWC-NH2 | C. textile | [5] |
| Glaconryphan-M | NγSγCPWHPWC-NH2 | C. marmoreus | [2] |
where the sequence abbreviations stand for:
- O = 4-trans-hydroxyproline,
- l = D-leucine, L = L-leucine,
- w = D-tryptophan, W = L-tryptophan,
- γ = gamma-carboxyglutamic acid,
- NH2 = C-terminal amidation
and the remainder of the letters refer to the standard one letter abbreviations for amino acids.
Mechanism of toxicity
The venom of cone snails cause paralysis of their fish prey. The molecular target has not been determined for all contryphan peptides, however it is known that contryphan-Vn is a Ca2+-dependent K+ channel modulator,[6] while glacontryphan-M is a L-type calcium channel blocker.[2]
See also
References
- ↑ PDB 1NXN; Eliseo T, Cicero DO, Romeo C, Schininà ME, Massilia GR, Polticelli F, Ascenzi P, Paci M (June 2004). "Solution structure of the cyclic peptide contryphan-Vn, a Ca2+-dependent K+ channel modulator". Biopolymers 74 (3): 189–98. doi:10.1002/bip.20025. PMID 15150794.
- ↑ 2.0 2.1 2.2 Hansson K, Ma X, Eliasson L, Czerwiec E, Furie B, Furie BC, Rorsman P, Stenflo J (2004). "The first gamma-carboxyglutamic acid-containing contryphan. A selective L-type calcium ion channel blocker isolated from the venom of Conus marmoreus". J. Biol. Chem. 279 (31): 32453–63. doi:10.1074/jbc.M313825200. PMID 15155730.
- ↑ 3.0 3.1 Jimenéz EC, Olivera BM, Gray WR, Cruz LJ (1996). "Contryphan is a D-tryptophan-containing Conus peptide". J. Biol. Chem. 271 (45): 28002–5. doi:10.1074/jbc.271.45.28002. PMID 8910408.
- ↑ Jimenez EC, Craig AG, Watkins M, Hillyard DR, Gray WR, Gulyas J, Rivier JE, Cruz LJ, Olivera BM (1997). "Bromocontryphan: post-translational bromination of tryptophan". Biochemistry 36 (5): 989–94. doi:10.1021/bi962840p. PMID 9033387.
- ↑ 5.0 5.1 5.2 5.3 5.4 Jacobsen R, Jimenez EC, Grilley M, Watkins M, Hillyard D, Cruz LJ, Olivera BM (1998). "The contryphans, a D-tryptophan-containing family of Conus peptides: interconversion between conformers". J. Pept. Res. 51 (3): 173–9. doi:10.1111/j.1399-3011.1998.tb01213.x. PMID 9531419.
- ↑ 6.0 6.1 Massilia GR, Schininà ME, Ascenzi P, Polticelli F (2001). "Contryphan-Vn: a novel peptide from the venom of the Mediterranean snail Conus ventricosus". Biochem. Biophys. Res. Commun. 288 (4): 908–13. doi:10.1006/bbrc.2001.5833. PMID 11688995.
- ↑ Jacobsen RB, Jimenez EC, De la Cruz RG, Gray WR, Cruz LJ, Olivera BM (1999). "A novel D-leucine-containing Conus peptide: diverse conformational dynamics in the contryphan family". J. Pept. Res. 54 (2): 93–9. doi:10.1034/j.1399-3011.1999.00093.x. PMID 10461743.
External links
- Contryphan at the US National Library of Medicine Medical Subject Headings (MeSH)