Clodronic acid
 | |
|---|---|
| Systematic (IUPAC) name | |
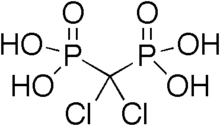
| (dichloro-phosphono-methyl)phosphonic acid | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Legal status | ? |
| Identifiers | |
| CAS number | 10596-23-3 |
| ATC code | M05BA02 |
| PubChem | CID 25419 |
| DrugBank | DB00720 |
| ChemSpider | 23731 |
| UNII | 0813BZ6866 |
| KEGG | D03545 |
| ChEBI | CHEBI:110423 |
| ChEMBL | CHEMBL12318 |
| Chemical data | |
| Formula | CH4Cl2O6P2 |
| Mol. mass | 244.892 g/mol |
| SMILES
| |
| |
| | |
Clodronic acid (INN) or clodronate disodium (USAN) is a first generation (non-nitrogenous) bisphosphonate. It is an anti-osteoporotic drug approved for the prevention and treatment of osteoporosis in post-menopausal women and men to reduce vertebral fractures, hyperparatiroidism, hypercalcemia in malignancy, multiple myeloma and fracture related pain because of its potent anti-inflammatory and analgesic effects shown as a reduction in inflammatory markers like IL-1β, IL-6, and TNF-alfa.
An Italian study compared the analgesic effect of clodronic acid versus acetaminophen in reumatic condition related pain. Study result show a reduction in pain in favor of clodronic acid that provided more analgesia than 3 grams/day of acetaminophen. Clodronate is also used in experimental medicine to selectively deplete macrophages.
Clodronic acid is approved for human use in Canada and Australia, the United Kingdom, where it is marketed as Bonefos, Loron, Clodron and in Italy as Clasteon, Difosfonal, Osteostab and several generics. In other countries is prescribed as a bone resorption inhibitor and antihypercalcemic agent.
| ||||||||||||||||