Clobutinol
From Wikipedia, the free encyclopedia
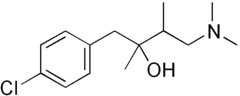 | |
|---|---|
| Systematic (IUPAC) name | |
| (RS)-1-(4-chlorophenyl)-4-dimethylamino-2,3-dimethyl-butan-2-ol | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Legal status | Withdrawn
(EU) |
| Routes | oral |
| Identifiers | |
| CAS number | 14860-49-2 |
| ATC code | R05DB03 |
| PubChem | CID 26937 |
| ChemSpider | 25085 |
| UNII | 1NY2IX043A |
| KEGG | D07716 |
| Chemical data | |
| Formula | C14H22ClNO |
| Mol. mass | 255.783 g/mol |
| SMILES
| |
| |
| | |
Clobutinol is a cough suppressant distributed by Boehringer-Ingelheim, Novartis's Hexal (Sandoz), Stada and possibly other companies.
Side effects and withdrawal
Studies in 2004 had indicated that clobutinol has the potential to prolong the QT interval.[1] Clobutinol was in 2007 determined to cause cardiac arrhythmia in some patients.[2]
Boehringer Ingelheim products containing clobutinol were voluntarily withdrawn from sale in Germany, and the rest of the world, on August 31, 2007.[3]
The approval for Germany and the EU was revoked in 2008.[4]
See also
References
- ↑ Bellocq, C.; Wilders, R.; Schott, J. J.; Louérat-Oriou, B.; Boisseau, P.; Le Marec, H.; Escande, D.; Baró, I. (2004). "A Common Antitussive Drug, Clobutinol, Precipitates the Long QT Syndrome 2". Molecular Pharmacology 66 (5): 1093–1102. doi:10.1124/mol.104.001065. PMID 15280442.
- ↑ "Clobutinol-haltige Arzneimittel: BfArM ordnet Widerruf der Zulassung an.". BfArM (German Federal Institute for Drugs and Medical Devices). 2007-08-31. "Clobutinol: BfArM orders cancellation of approval"
- ↑ "Boehringer Ingelheim voluntarily withdraws its clobutinol containing medications.". Boehringer Ingelheim. 2007-08-31.
- ↑ "Cancellation of approval" (pdf). BfArM (German Federal Institute for Drugs and Medical Devices). 2008-06-06. "Die Zulassungen für die o.g. Arzneimittel werden mit sofortiger Wirkung widerrufen."
| ||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.