Classical complement pathway

The Classical pathway of activation of the complement system is a group of blood proteins that mediate the specific antibody response. The main activators of the Classical Pathway are antigen-antibody complexes.
Initiation
It is triggered by antigen-bound antibody molecules. (The only antibodies capable of binding are two units of IgG or one IgM, although IgM is more effective at activating complement. IgG4 cannot bind, but the other three IgG types can.) It is the binding of Fc region to the C1 component that initiates this pathway. The classical pathway can also be triggered by binding of C1 to some types of structures on the target microbe. Also, it can be triggered by C-Reactive Protein binding to microbic polysaccharides such as phosphocholine.[1]
C1 and its subunits
This initial enzyme, C1, is a complex formed through a calcium-dependent association between two reversibly interacting subunits, C1q and C1r2s2. In the absence of an immune response, approximately 70% of C1 exists as a complex between these two subunits. C1 occurs in serum as a proenzyme that tends to undergo autoactivation (in which the two subunits are bound together) but is strictly controlled by C1-inhibitor (C1-In or C1 esterase). Upon the binding of C1 to immune complexes by virtue of the affinity of C1q for immunoglobulins (to be specific, IgM and IgG), the controlling action of C1-In is overcome, and C1q effects activation of C1r2s2.
C1q possesses no intrinsic catalytic activity, but, when any of several activators bind to the C1q subcomponent of C1, the homologous C1r and C1s subcomponents are converted into catalytically active species, namely C1r* and C1s*, triggering the first step of the classical pathway of complement activation.
Thus, on binding to immune complexes through C1q, the subunits of C1 become firmly associated and autoactivation commences even in the presence of the C1-In.
Steps leading to C3-convertase
At first, a conformational change in C1r occurs, followed by proteolytic activation, which results in the cleavage of all four polypeptide chains of C1r2s2.
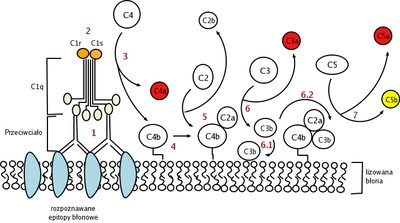
The two activated C1s subunits cleave C4 into C4a and C4b. C4a is released while C4b binds to the membrane. C4b then binds to another protein C2 and stimulates C1s to cleave it into C2a and C2b. C2b is released while C2a binds to C4b to form C4b2a. C4b2a is able to catalyse the assembly of the C3-convertase.
See also
External links
- Immunology at MCG 2/fixation
References
- ↑ Carolyn Mold; Henry Gewurz; Terry W Du Clos (May 1999). "Regulation of complement activation by C-reactive protein". Immunopharmacology 42 (1–3): 23–30. doi:10.1016/S0162-3109(99)00007-7.
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||