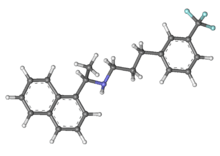
Cinacalcet
 | |
|---|---|
 | |
| Systematic (IUPAC) name | |
| (R)-N-[1-(1-naphthyl)ethyl]-3- [3-(trifluoromethyl)phenyl]propan-1-amine | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a605004 |
| Licence data | EMA:Link, US FDA:link |
| Pregnancy cat. | B3 (AU) C (US) |
| Legal status | ℞ Prescription only |
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 20 to 25% Increases if taken with food |
| Protein binding | 93 to 97% |
| Metabolism | Hepatic (CYP3A4-, CYP2D6- and CYP1A2-mediated) |
| Half-life | 30 to 40 hours |
| Excretion | Renal (80%) and fecal (15%) |
| Identifiers | |
| CAS number | 226256-56-0 |
| ATC code | H05BX01 |
| PubChem | CID 156419 |
| IUPHAR ligand | 3308 |
| DrugBank | DB01012 |
| ChemSpider | 137743 |
| UNII | UAZ6V7728S |
| KEGG | D03504 |
| ChEBI | CHEBI:48390 |
| ChEMBL | CHEMBL1201284 |
| Chemical data | |
| Formula | C22H22F3N |
| Mol. mass | 357.412 g/mol |
| SMILES
| |
| |
| | |
Cinacalcet (INN) is a drug that acts as a calcimimetic (i.e. it mimics the action of calcium on tissues) by allosteric activation of the calcium-sensing receptor that is expressed in various human organ tissues. It is sold by Amgen under the trade name Sensipar in North America and Australia and as Mimpara in Europe. Cinacalcet is used to treat secondary hyperparathyroidism (elevated parathyroid hormone levels), a consequence of end-stage renal disease.[1] Cinacalcet is also indicated for the treatment of hypercalcemia in patients with parathyroid carcinoma.[2]
Clinical trials conducted in the United States by Amgen to determine whether the drug is safe for children were halted by the FDA in February 2013 following the death of a 14-year-old patient.[3]
Clinical uses
Cinacalcet is indicated for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease on dialysis and hypercalcemia in patients with parathyroid carcinoma.
Synthesis
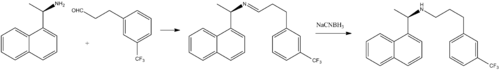
B.C. Van Wagenen, S.T. Moe, M.F. Balandrin, E.G. DelMar, E.F. Nemeth, U.S. Patent 6,211,244 (2001).
References
- ↑ Torres PU (2006). "Cinacalcet HCl: a novel treatment for secondary hyperparathyroidism caused by chronic kidney disease". Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation 16 (3): 253–8. doi:10.1053/j.jrn.2006.04.010. PMID 16825031.
- ↑ "Sensipar for Parathyroid Carcinoma". Amgen. 2009. Retrieved 25 September 2009.
- ↑ Edney, Anna (February 26, 2013). "Amgen Pediatric Trials of Sensipar Halted by FDA After Death". Businessweek.
External links
- Sensipar website run by Amgen
- Prescribing information (package insert)
- Cinacalcet - Medlineplus.org
- Cinacalcet - Drug Digest.
| |||||||||||||