Chugaev elimination
From Wikipedia, the free encyclopedia
The Chugaev elimination is a chemical reaction that involves the elimination of water from alcohols to produce alkenes. The intermediate is a xanthate. It is named for its discoverer, the Russian chemist Lev Aleksandrovich Chugaev.
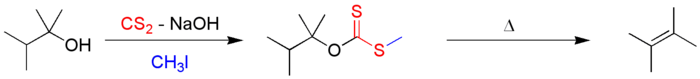
In the first step, a potassium xanthate is formed out of the alkoxide and carbon disulfide (CS2). With iodomethane, it is transformed into a xanthate.
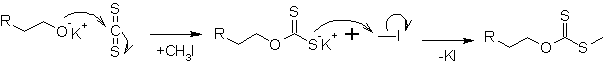
At about 200 °C, the alkene is formed by a syn-elimination. In a 6-membered cyclic transition state the hydrogen atom is moved from the β-C-atom to the sulfur. The side product decomposes to carbonyl sulfide (OCS) and methanethiol.
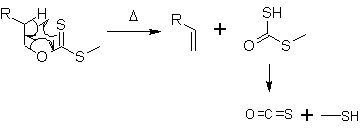
References
- Latscha, Hans P. (2002). Chemie-Basiswissen. Berlin: Springer.
- L. Tschugaeff (1900). "Ueber das Thujen, ein neues bicyclisches Terpen". Berichte der deutschen chemischen Gesellschaft 33 (3): 3118–3126. doi:10.1002/cber.19000330363.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.