Chromium hydride
Chromium hydride, also known as chromium–hydrogen alloy, is an alloy of chromium, hydrogen, and possibly other elements. An alloying element intentionally added to modify the characteristics of chromium hydride is titanium.[1]
Hydrogen reduces the stress necessary to force dislocations in the chromium atom crystal lattice to slide past one another. Varying the amount of hydrogen and other alloying elements and the form of their presence in the chromium hydride (solute elements, precipitated phase) controls qualities such as the hardness, ductility, and tensile strength of the resulting chromium hydride. Chromium hydride with increased hydrogen content can be made softer and more ductile than chromium.
Chromium hydride phases
At room temperature, the most stable form of chromium is the body-centered cubic (bcc) structure α-chromium. As the hydrogen concentration in chromium increases the following are observed:[2]-
- For compositions between CrH0.5 and CrH the metal lattice changes to hexagonal close packed (hcp), and the hydrogen atoms are in octahedral positions in the lattice
- For compositions between CrH and CrH2 the metal lattice structure changes to face centred cubic (fcc) with hydrogen atoms again occupying octahedral positions.
Material properties
Other materials are often added added to the chromium/hydrogen mixture to produce chromium hydride with desired properties. Titanium in chromium hydride make the β-titanium form of the chromium-hydrogen solution more stable.
Even in the narrow range of concentrations that make up chromium hydride, mixtures of hydrogen and chromium can form a number of different structures, with very different properties. Understanding such properties is essential to making quality chromium hydride. At room temperature, the most stable form of chromium is the body-centered cubic (BCC) structure α-chromium. It can occur as a dull brown or dark grey solid in two different crystalline forms: face centred cubic with formula CrH~2 or a close packed hexagonal solid with formula CrH~1. Chromium hydride is important in chrome plating, being an intermediate in the formation of the chromium plate.
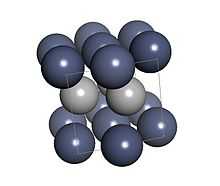

An apparent unusual allotrope of chromium in a hexagonal crystal form was investigated by Ollard and Bradley by X-ray crystallography; however they failed to notice that it contained hydrogen.[3] The hexagonal close packed crystalline substance they discovered actually contains CrHx with x between 0.5 and 1.[4] The lattice for the hexagonal form had unit cell dimensions a=0.271 nm and c=0.441 nm.[5] The crystal form has been described as anti-NiAs structure and is known as the β-phase.[6] Also known as ε-CrH, the space group is Fm3m with hydrogen only in octahedral sites.[7]
A face-centered cubic (fcc) phase of chromium hydride can also be produced when chromium is electrodeposited. Cloyd A. Snavely used chromate in sugar syrup cooled to about 5°C and with a current density of 1290 amperes per square meter. The unit cell dimension in the material was 0.386 nm. The material is brittle and easily decomposed by heat. The composition is CrHx, with x between 1 and 2.[4] For current density above 1800 amps per square meter and at low temperatures, the hexagonal close-packed form was made, but if the current was lower or the temperature was higher, then regular body-centred cubic chromium metal was deposited.[8] The condition for preferring the formation of face-centered cubic chromium hydride is a high pH.[5] The fcc form of CrH has hydrogen atoms in octahedaral sites in the P63/mmc spacegroup.[7]

Face-centred cubic CrH had the composition CrH1.7.[5] But in theory it would be CrH2 if the substance was pure and all the tetrahedral sites were occupied by hydrogen atoms. The solid substance CrH2 appears as a dull grey or brown colour. Its surface is easily scratched, but that is due to the brittleness of the hydride.[5]
Face centred cubic chromium hydride also forms temporarily when chromium metal is etched with hydrochloric acid.[9]
The hexagonal form spontaneously changes to normal chromium in 40 days, whereas the other form (face-centred cubic) changes to the body-centred cubic form of chromium in 230 days at room temperature. Ollard already noticed that hydrogen is evolved during this transformation, but was not sure that the hydrogen was an essential component of the substance, as electrodeposited chromium usually contained hydrogen. Colin G Fink observed that if the hexagonal form was heated in a flame that the hydrogen would quickly burn off.[8]
Electroplating chromium metal from a chromate solution involves the formation of chromium hydride. If the temperature is high enough the chromium hydride rapidly decomposes as it forms, yielding microcrystalline body-centred cubic chromium. Therefore, to ensure that the hydride decomposes sufficiently rapidly and smoothly, chromium must be plated at a suitably high temperature (roughly 60C to 75C, depending on conditions). As the hydride decomposes, the plated surface cracks. The cracking can be controlled and there may be up to 40 cracks per millimeter. Substances on the plating surface, mostly chromium sesquioxide, are sucked into the cracks as they form. The cracks heal over and newer electroplated layers will crack differently. When observed with a microscope the electroplated chromium will appear to be in the form of crystals with 120° and 60° angles, but these are the ghosts of the original hydride crystals; the actual crystals that finally form in the coating are much smaller and consist of body centred cubic chromium.[5]
Superhexagonal chromium hydride has also been produced by exposing chromium films to hydrogen under high pressure and temperature.[10]
In 1926 T. Weichselfelder and B. Thiede claimed to have prepared solid chromium trihydride by reacting hydrogen with chromium chloride and phenyl magnesium bromide in ether, forming a black precipitate.[11][12]
Solid hexagonal CrH can burn in air with a bluish flame. It is ignitable with a burning match.[13]
References
- ↑ Johnson, John R.; Reilly, James J. (November 1978). "Reaction of hydrogen with the low-temperature form (C15) of titanium-chromium (TiCr2)". Inorganic Chemistry 17 (11): 3103–3108. doi:10.1021/ic50189a027. Retrieved 30 March 2013.
- ↑ Bradley, A. J.; E. F. Ollard (1926). "Allotropy of Chromium". Nature 117 (2934): 122–122. doi:10.1038/117122b0. ISSN 0028-0836.
- ↑ Poźniak-Fabrowska, J; B Nowak, M Tkacz (2001). "Magnetic properties of cubic and hexagonal chromium hydrides: a comparison of the magnetic susceptibility with the 53Cr NMR Knight shift". Journal of Alloys and Compounds 322 (1-2): 82–88. doi:10.1016/S0925-8388(01)01266-X. ISSN 0925-8388.
- ↑ 7.0 7.1 Antonov, V.E.; A.I. Beskrovnyy, V.K. Fedotov, A.S. Ivanov, S.S. Khasanov, A.I. Kolesnikov, M.K. Sakharov, I.L. Sashin, M. Tkacz (2007). "Crystal structure and lattice dynamics of chromium hydrides". Journal of Alloys and Compounds 430 (1-2): 22–28. doi:10.1016/j.jallcom.2006.05.021. ISSN 0925-8388.
- ↑ 8.0 8.1 Sasaki, Kumazo; Sinkiti Sekito (24 February 1931). "Three Crystalline Modifications of Electrolytic Chromium". Journal of The Electrochemical Society 59 (1): 437–444. doi:10.1149/1.3497824. full text available
- ↑ Smith, W. H. (1956). "The Fracture of Brittle Chromium by Acid Etching". Journal of The Electrochemical Society 103 (1): 51. doi:10.1149/1.2430232. ISSN 0013-4651.
- ↑ Pan, Y; M Takeo, J Dash (1993). "Formation of superhexagonal chromium hydride by exposure of chromium thin films to high-temperature, high-pressure hydrogen in a ballistic compressor". International Journal of Hydrogen Energy 18 (6): 491–504. doi:10.1016/0360-3199(93)90006-V. ISSN 0360-3199.
- ↑ mellor. "The Chemical Properties of Chromium". A Comprehensive Treatise on Inorganic and Theoretical Chemistry. p. 160.
- ↑ Weichselfelder, Theodor; Bruno Thiede (1926). "Über die Hydride der Metalle Nickel, Kobalt, Eisen und Chrom". Justus Liebigs Annalen der Chemie 447 (1): 64–77.
- ↑ Raub, Christoph J. (September 1993). "Hydrogen in Electrodeposits: of Decisive Importance, But Much Neglected". Plating and Surface Finishing: 35.
Additional reading
- Wolf, G. (1971). "Specific heat of chromium hydride CrHx from 11 to 300 °K". Physica Status Solidi (a) 5 (3): 627–632. doi:10.1002/pssa.2210050312. ISSN 0031-8965.
- Khan, H.R; Ch.J Raub (1976). "Properties of chromium hydride". Journal of the Less Common Metals 49: 399–406. doi:10.1016/0022-5088(76)90051-5. ISSN 0022-5088.
- Kerle, Bettina; Mathias Opper, Sigrid Volk (6 September 2000). "Hexavalent Chromium Processes". SurTec GmbH. Retrieved 1 August 2012.
- Stock, Allen D.; Kenneth I. Hardcastle (1970). "Phase and composition analysis of chromium hydride". Journal of Inorganic and Nuclear Chemistry 32 (4): 1183–1186. doi:10.1016/0022-1902(70)80113-0. ISSN 0022-1902.
- Khan, H.R.; A. Knödler, Ch.J. Raub, A.C. Lawson (1974). "Electrical and magnetic properties of chromium hydride". Materials Research Bulletin 9 (9): 1191–1197. doi:10.1016/0025-5408(74)90037-3. ISSN 0025-5408.
- Venkatraman, M; J.P Neumann (1991). "The cr-h (chromium-hydrogen) system". Journal of Phase Equilibria 12 (6): 672–677. doi:10.1007/BF02645169. ISSN 1054-9714.