Chlorosulfonyl isocyanate
| Chlorosulfonyl isocyanate | |
|---|---|
 | |
 | |
| IUPAC name Chlorosulfonyl isocyanate | |
| Other names N-Carbonylsulfamyl chloride | |
| Identifiers | |
| CAS number | 1189-71-5 |
| PubChem | 70918 |
| ChemSpider | 64080 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | CNClO3S |
| Molar mass | 141.53 g/mol |
| Appearance | colorless liquid |
| Density | 1.626 g/cm3 |
| Melting point | -44 °C |
| Boiling point | 107 °C |
| Solubility in water | decomposition |
| Solubility in other solvents | Chlorocarbons MeCN |
| Refractive index (nD) | 1.447 |
| Structure | |
| Molecular shape | tetrahedral at S |
| Hazards | |
| MSDS | "External MSDS" |
| R-phrases | R14 R20 R24/25 R29 R34 R42/43[1] |
| S-phrases | (S1/2) S8 S24 S26 S30 S36/37/39 S38 S45 [1] |
| Main hazards | toxic, corrosive, flammable, reacts violently with water |
| NFPA 704 |
 1
3
2
|
| Related compounds | |
| Related compounds | Thionyl chloride Cyanogen bromide Phosphoryl chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Chlorosulfonyl isocyanate is the chemical compound ClSO2NCO, known as CSI. This compound is a versatile reagent in organic synthesis.
Preparation, structure, handling
CSI is prepared by treating cyanogen chloride with sulfur trioxide, the product being distilled directly from the reaction mixture.[2]
- SO3 + ClCN → ClSO2NCO
In this transformation, both the carbon and the nitrogen termini of CN are functionalized.
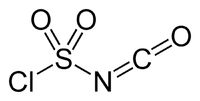
The structure of CSI is represented as ClS(O)2-N=C=O. It consists of two electron-withdrawing components, the chlorosulfonyl group (SO2Cl) and the isocyanate group (-N=C=O). Because of its resulting electrophilicity, the use of CSI in chemical synthesis requires relatively inert solvents such as chlorocarbons, acetonitrile, and ethers.[3]
Uses
The molecule has two electrophilic sites, the carbon and the S(VI) center.[4]
CSI has been employed for the preparation of β-lactams, some of which are medicinally important. Thus, alkenes undergo a [2+2]-cycloaddition to give the sulfonamide. The SO2Cl group can be removed simply by hydrolysis, leaving the secondary amide.[5] Other reactions of CSI:
- Cycloaddition to alkynes to give 1,2,3-oxathiazine-2,2-dioxide-6-chlorides.
- Conversion of primary alcohols to carbamates.[6]
- Conversion of carboxylic acids and the acid chlorides into nitriles.
- Preparation of N,N-disubstituted sulfamides, R2NSO2NH2
Safety considerations
CSI is toxic, corrosive and reacts violently with water. It cannot be stored in glass-stoppered flasks, requiring instead polyethylene bottles.
References
- ↑ 1.0 1.1 http://fscimage.fishersci.com/msds/54436.htm
- ↑ Graf, R. "Chlorosulfonyl Isocyanate" Organic Syntheses, Collected Volume 5, pages 226ff.
- ↑ Miller, M. J.; Ghosh, M.; Guzzo, P. R.; Vogt, P. F.; Hu, J.; Filzen, G. F.; Geyer, A. G. "Chlorosulfonyl Isocyanate" in "Encyclopedia of Reagents for Organic Synthesis" 2005 John Wiley & Sons: New York.
- ↑ D. N. Dhar, K. S. K. Murthy "Recent Advances in the Chemistry of Chlorosulfonyl Isocyanate" Synthesis 1986; pages 437-449.
- ↑ Cremlyn, R. J. “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). ISBN 0-471-95512-4
- ↑ Burgess, E. M.; Penton, Jr., H. R.; Taylor, E. A.; Williams, W. M. "Conversion of Primary Alcohols to Urethanes via the Inner Salt of Triethylammonium Hydroxide: Methyl (Carboxylsulfamoyl) Triethylammonium Hydroxide Methyl n-Hexylcarbamate" Organic Syntheses, Coll. Vol. 6, p.788