Chloroacetaldehyde
| Chloroacetaldehyde | |
|---|---|
 |
 |
| IUPAC name Chloroacetaldehyde | |
| Systematic name Chloroethanal | |
| Identifiers | |
| CAS number | 107-20-0 |
| PubChem | 33 |
| ChemSpider | 32 |
| UNII | CF069F5D9C |
| EC number | 203-472-8 |
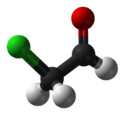
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
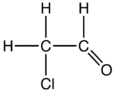
| Molecular formula | C2H3ClO |
| Molar mass | 78.50 g mol-1 |
| Appearance | Colourless liquid |
| Odor | accrid, penetrating |
| Density | 1.117 g/mL |
| Melting point | −16.3 °C; 2.7 °F; 256.8 K |
| Boiling point | 85–85.5 °C |
| Solubility in water | soluble |
| Solubility | soluble in acetone, methanol |
| Hazards | |
| Main hazards | alkylating agent |
| Flash point | 87.7 °C (189.9 °F) (closed cup) |
| Related compounds | |
| Related compounds | 2-chloroethanol, Chloroacetic acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Chloroacetaldehyde is the organic compound with the formula ClCH2CHO. Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent. The compound is not normally encountered in the anhydrous form, but rather as the hydrate (acetal), ClCH2CH(OH)2. Chloroacetaldehyde is a useful intermediate in the synthesis, e.g. of 2-aminothiazole or many pharmaceutical compounds. Another use is to facilitate bark removal from tree trunks.
Synthesis and reactions
The hydrate of chloroacetaldehyde is produced by the oxidation of aqueous vinyl chloride using chlorine:
- ClCH=CH2 + Cl2 + H2O → ClCH2CHO + 2 HCl
It can also be prepared from vinyl acetate.[1]
Being bifunctional, chloroacetaldehyde is a versatile precursor to many heterocyclic compounds. It condenses with thiourea derivatives to give aminothiazoles. This reaction was once important as a precursor to sulfathiazole, one of the first sulfa drugs.[1]
Environmental aspects
Chloroacetaldehyde is a metabolite in the degradation of 1,2-dichloroethane, which initially converts to chloroethanol. This metabolic pathway is topical since billions of kilograms of 1,2-dichloroethane have been produced as a precursor to vinyl chloride.[2]
Safety
Based on data collected from human studies in 1962, exposures to 45 ppm of chloroacetaldehyde were found to be very disagreeable and caused conjunctival irritation to the subjects.[3] The Occupational Safety and Health Administration established a permissible exposure limit at a ceiling of 1 ppm (3 mg/m3) for exposures to chloroacetaldehyde.[4]
References
- ↑ 1.0 1.1 Reinhard Jira, Erwin Kopp, Blaine C. McKusick, Gerhard Röderer, Axel Bosch, Gerald Fleischmann “Chloroacetaldehydes“ in Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_527.pub2
- ↑ Janssen, D. B.; van der Ploeg, J. R. and Pries, F., "Genetics and biochemistry of 1,2-dichloroethane degradation", Biodegradation, 1994, 5, 249-57.doi:10.1007/BF00696463
- ↑ Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs)
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards