Chlorine oxide
From Wikipedia, the free encyclopedia
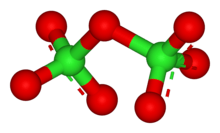
Dichlorine heptoxide (Cl2O7)
Chlorine and oxygen can bond in many different ways:
- chlorine monoxide (ClO), chlorine(II) oxide
- chlorine dioxide (ClO2), chlorine(IV) oxide
- chlorine trioxide (ClO3), chlorine(VI) oxide
- dichlorine monoxide (Cl2O), chlorine(I) oxide
- dichlorine dioxide (Cl2O2), chlorine(I) peroxide
- dichlorine trioxide (Cl2O3), chlorine(I,V) oxide
- dichlorine tetroxide, also known as chlorine perchlorate, (ClOClO3), chlorine(I,VII) oxide
- dichlorine hexoxide (Cl2O6), chlorine(V,VII) oxide
- dichlorine heptoxide (Cl2O7), chlorine(VII) oxide
- chlorine tetroxide, the peroxide ((OClO3)2), chlorine(VII) oxide peroxide
In addition, a number of ions are chlorine oxides:
- chloryl (ClO2+)
- perchloryl (ClO3+)
- hypochlorite (ClO-)
- chlorite (ClO2-)
- chlorate (ClO3-)
- perchlorate (ClO4-)
See also
| |
This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.