Chirality

Chirality /kaɪˈrælɪtiː/ is a property of asymmetry important in several branches of science. The word chirality is derived from the Greek, χειρ (kheir), "hand", a familiar chiral object.
An object or a system is chiral if it is not identical to its mirror image, that is, it cannot be superposed onto it. A chiral object and its mirror image are called enantiomorphs (Greek opposite forms) or, when referring to molecules, enantiomers. A non-chiral object is called achiral (sometimes also amphichiral) and can be superposed on its mirror image.
The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894.[1]
I call any geometrical figure, or group of points, 'chiral', and say that it has chirality if its image in a plane mirror, ideally realized, cannot be brought to coincide with itself.
Human hands are perhaps the most universally recognized example of chirality: The left hand is a non-superimposable mirror image of the right hand; no matter how the two hands are oriented, it is impossible for all the major features of both hands to coincide.[2] This difference in symmetry becomes obvious if someone attempts to shake the right hand of a person using his left hand, or if a left-handed glove is placed on a right hand. In mathematics chirality is the property of a figure that is not identical to its mirror image.
Mathematics
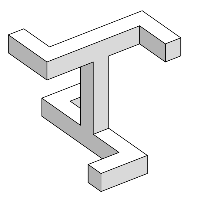
In mathematics, a figure is chiral (and said to have chirality) if it cannot be mapped to its mirror image by rotations and translations alone. For example, a right shoe is different from a left shoe, and clockwise is different from anti-clockwise.
A chiral object and its mirror image are said to be enantiomorphs. The word enantiomorph stems from the Greek ἐναντίος (enantios) 'opposite' + μορφή (morphe) 'form'. A non-chiral figure is called achiral or amphichiral.
The helix (and by extension a spun string, a screw, a propeller, etc.) and Möbius strip are chiral two-dimensional objects in three-dimensional ambient space. The J, L, S and Z-shaped tetrominoes of the popular video game Tetris also exhibit chirality, but only in a two-dimensional space.
Many other familiar objects exhibit the same chiral symmetry of the human body, such as gloves, glasses (where two lenses differ in prescription), and shoes. A similar notion of chirality is considered in knot theory, as explained below.
Some chiral three-dimensional objects, such as the helix, can be assigned a right or left handedness, according to the right-hand rule.
Geometry
In geometry a figure is achiral if and only if its symmetry group contains at least one orientation-reversing isometry. In two dimensions, every figure that possesses an axis of symmetry is achiral, and it can be shown that every bounded achiral figure must have an axis of symmetry. In three dimensions, every figure that possesses a plane of symmetry or a center of symmetry is achiral. There are, however, achiral figures lacking both plane and center of symmetry.
Knot theory
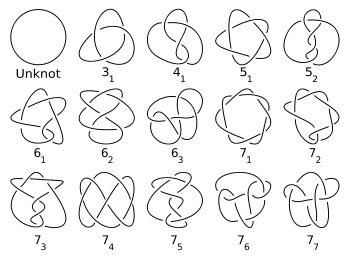
A knot is called achiral if it can be continuously deformed into its mirror image, otherwise it is called chiral. For example the unknot and the figure-eight knot are achiral, whereas the trefoil knot is chiral.
Physics
In physics, chirality may be found in the spin of a particle, which may be used to define a handedness (aka chirality) for that particle. A symmetry transformation between the two is called parity. Invariance under parity by a Dirac fermion is called chiral symmetry.
Electro-magnetism
Electromagnetic wave propagation as handedness is wave polarization and described in terms of helicity (occurs as a helix). Polarization of an electromagnetic wave, is the property that describes the orientation, i.e., the time-varying, direction (vector), and amplitude of the electric field vector. For a depiction, see the image to the right.
Chemistry
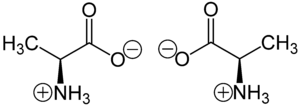
A chiral molecule is a type of molecule that has a non-superposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom.[3][4]
The term chiral in general is used to describe an object that is non-superposable on its mirror image.[5]
In chemistry, chirality usually refers to molecules. Two mirror images of a chiral molecule are called enantiomers or optical isomers. Pairs of enantiomers are often designated as "right-" and "left-handed."
Molecular chirality is of interest because of its application to stereochemistry in inorganic chemistry, organic chemistry, physical chemistry, biochemistry, and supramolecular chemistry.
Biology

In anatomy, chirality is found in the imperfect mirror-image symmetry of many kinds of animal bodies. Organisms such as gastropods exhibit chirality in their coiled shells, resulting in an asymmetrical appearance. Over 90% of gastropod species [6] have dextral (right-handed) shells in their coiling, but a small minority of species and genera are virtually always sinistral (left-handed). A very few species (for example Amphidromus perversus[7]) show an equal mixture of dextral and sinistral individuals.
In humans, chirality (also referred to as handedness or laterality) is an attribute of humans defined by their unequal distribution of fine motor skill between the left and right hands. An individual who is more dexterous with the right hand is called right-handed, and one who is more skilled with the left is said to be left-handed. Chirality is also seen in the study of facial asymmetry.
In flatfish, the Summer flounder or fluke are left-eyed, while halibut are right-eyed.
See also
- Orientation (mathematics)
- Stereochemistry
- Right-hand rule
- Handedness
- Asymmetry
References
- ↑ Sir William Thomson Lord Kelvin (1894). The Molecular Tactics of a Crystal. Clarendon Press.
- ↑ Georges Henry Wagnière, On Chirality and the Universal Asymmetry: Reflections on Image and Mirror Image (2007).
- ↑ Organic Chemistry (4th Edition) Paula Y. Bruice.
- ↑ Organic Chemistry (3rd Edition) Marye Anne Fox ,James K. Whitesell.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "chirality".
- ↑ Schilthuizen M. & Davison A. (2005). "The convoluted evolution of snail chirality". Naturwissenschaften 92(11): 504–515. doi:10.1007/s00114-005-0045-2.
- ↑ Amphidromus perversus (Linnaeus, 1758)
External links
| Look up chirality in Wiktionary, the free dictionary. |
