Chemiluminescence

Chemiluminescence (sometimes "chemoluminescence") is the emission of light (luminescence), as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊,
- [A] + [B] → [◊] → [Products] + light
For example, if [A] is luminol and [B] is hydrogen peroxide in the presence of a suitable catalyst we have:
- luminol + hydrogen peroxide → 3-APA[◊] → 3-APA + light
where:
- where 3-APA is 3-aminophthalate
- 3-APA[◊] is the vibronic excited state fluorescing as it decays to a lower energy level.
The decay of this excited state[◊] to a lower energy level causes light emission. In theory, one photon of light should be given off for each molecule of reactant. This is equivalent to Avogadro's number of photons per mole of reactant. In actual practice, non-enzymatic reactions seldom exceed 1% QC, quantum efficiency.
In a chemical reaction, reactants collide to form a transition state, the enthalpic maximum in a reaction coordinate diagram, which proceeds to the product. Normally, reactants form products of lesser chemical energy. The difference in energy between reactants and products, represented as 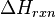 , is turned into heat, physically realized as excitations in the vibrational state of the normal modes of the product. Since vibrational energy is generally much greater than the thermal agitation, it rapidly disperses in the solvent through molecular rotation. This is how exothermic reactions make their solutions hotter. In a chemiluminescent reaction, the direct product of a reaction is an excited electronic state, which then decays into an electronic ground state through either fluorescence or phosphorescence, depending partly on the spin state of the electronic excited state formed.
, is turned into heat, physically realized as excitations in the vibrational state of the normal modes of the product. Since vibrational energy is generally much greater than the thermal agitation, it rapidly disperses in the solvent through molecular rotation. This is how exothermic reactions make their solutions hotter. In a chemiluminescent reaction, the direct product of a reaction is an excited electronic state, which then decays into an electronic ground state through either fluorescence or phosphorescence, depending partly on the spin state of the electronic excited state formed.
Chemiluminescence differs from fluorescence in that the electronic excited state is derived from the product of a chemical reaction rather than the more typical way of creating electronic excited states, namely absorption. It is the antithesis of a photochemical reaction, in which light is used to drive an endothermic chemical reaction. Here, light is generated from a chemically exothermic reaction.
A standard example of chemiluminescence in the laboratory setting is the luminol test. Here, blood is indicated by luminescence upon contact with iron in hemoglobin. When chemiluminescence takes place in living organisms, the phenomenon is called bioluminescence. A light stick emits light by chemiluminescence.
Liquid-phase reactions
- Luminol in an alkaline solution with hydrogen peroxide in the presence of iron or copper,[1] or an auxiliary oxidant,[2] produces chemiluminescence. The luminol reaction is
- luminol + H2O2 → 3-APA[◊] → 3-APA + light
Gas-phase reactions
- One of the oldest known chemoluminescent reactions is that of elemental white phosphorus oxidizing in moist air, producing a green glow. This is a gas-phase reaction of phosphorus vapor, above the solid, with oxygen producing the excited states (PO)2 and HPO.[3]
- Another gas phase reaction is the basis of nitric oxide detection in commercial analytic instruments applied to environmental air-quality testing. Ozone is combined with nitric oxide to form nitrogen dioxide in an activated state.
- NO+O3 → NO2[◊]+ O2
- The activated NO2[◊] luminesces broadband visible to infrared light as it reverts to a lower energy state. A photomultiplier and associated electronics counts the photons that are proportional to the amount of NO present. To determine the amount of nitrogen dioxide, NO2, in a sample (containing no NO) it must first be converted to nitric oxide, NO, by passing the sample through a converter before the above ozone activation reaction is applied. The ozone reaction produces a photon count proportional to NO that is proportional to NO2 before it was converted to NO. In the case of a mixed sample that contains both NO and NO2, the above reaction yields the amount of NO and NO2 combined in the air sample, assuming that the sample is passed through the converter. If the mixed sample is not passed through the converter, the ozone reaction produces activated NO2[◊] only in proportion to the NO in the sample. The NO2 in the sample is not activated by the ozone reaction. Though unactivated NO2 is present with the activated NO2[◊], photons are emitted only by the activated species that is proportional to original NO. Final step: Subtract NO from (NO + NO2) to yield NO2[4]
Enhanced chemiluminescence
Enhanced chemiluminescence is a common technique for a variety of detection assays in biology. A horseradish peroxidase enzyme (HRP) is tethered to an antibody that specifically recognizes the molecule of interest. This enzyme complex then catalyzes the conversion of the enhanced chemiluminescent substrate into a sensitized reagent in the vicinity of the molecule of interest, which on further oxidation by hydrogen peroxide, produces a triplet (excited) carbonyl, which emits light when it decays to the singlet carbonyl. Enhanced chemiluminescence allows detection of minute quantities of a biomolecule. Proteins can be detected down to femtomole quantities,[5] well below the detection limit for most assay systems.
Applications
- Gas analysis: for determining small amounts of impurities or poisons in air. Other compounds can also be determined by this method (ozone, N-oxides, S-compounds). A typical example is NO determination with detection limits down to 1 ppb
- Analysis of inorganic species in liquid phase
- Analysis of organic species: useful with enzymes, where the substrate is not directly involved in chemiluminescence reaction, but the product is
- Detection and assay of biomolecules in systems such as ELISA and Western blots
- DNA sequencing using pyrosequencing
- Lighting objects. Chemiluminescence kites,[6] emergency lighting, glow sticks (party decorations).
- Combustion analysis: Certain radical species (such as CH* and OH*) give off radiation at specific wavelengths. The heat release rate is calculated by measuring the amount of light radiated from a flame at those wavelengths. Chemiluminescence as a Combustion Diagnostic[7]
- Children's toys
Biological Applications
Biological Applications
- Chemiluminescence has been applied by forensic scientists to solve crimes. In this case, they use luminol and hydrogen peroxide. The iron from the blood acts as a catalyst and reacts with the luminol and hydrogen peroxide to produce blue light for about 30 seconds. Because only a small amount of iron is required for chemiluminescence, even the most trace amounts of blood can be observed.
- In cancer research, the gene that gives fireflies it glows along with the protein luciferin is used to test the effectiveness of cancer drugs that choke of a tumor’s blood supply. This form of bioluminescence imaging allows scientists to test drugs in the pre-clinical stages cheaply.
- Also, scientist have been investigating aequorin, a protein found in certain jellyfish, which produces blue light in the presence of calcium). It can be used in molecular biology to assess calcium levels in cells.
- What these biological reactions have in common is their use of ATP (adenosine triphosphate) as an energy source. Though the structure of the molecules that produce luminescence is different for each species, they are given the generic name of luciferin.
- One example is fireflies’ luciferin can be oxidized, as demonstrated by the diagram to produce an excited complex. Once it falls back down to a ground state a photon is released. It is very similar to the reaction with luminol.
- Luciferase = Luciferin + O2 → Oxyluciferin + Light
- Many organisms have evolved to produce light. Some vary in color. At the molecular level, the difference in color arises from the degree of conjugation of the molecule, when an electron drops down from the excited state to the ground state. Deep sea organisms have evolved to produce light lure and catch prey, as camouflage or attract others. Some bacteria even use bioluminescence to communicate. The common color for the light emitted by these animals are blue and green because they have shorter wavelength than red and can transmit more easily in water.
Notice that chemiluminescence is different from fluorescence. Hence the application of fluorescent proteins such as Green fluorescent protein can not be attributed to the biological applications of chemiluminescence.
See also
References
- ↑ "Luminol chemistry laboratory demonstration". Retrieved 2006-03-29.
- ↑ "Investigating luminol" (PDF). Salters Advanced Chemistry. Retrieved 2006-03-29.
- ↑ Rauhut, Michael M. (1985), Chemiluminescence. In Grayson, Martin (Ed) (1985). Kirk-Othmer Concise Encyclopedia of Chemical Technology (3rd ed), pp 247 John Wiley and Sons. ISBN 0-471-51700-3
- ↑ Air Zoom | Glowing with Pride. Fannation.com. Retrieved on 2011-11-22.
- ↑ Enhanced CL review. Biocompare.com (2007-06-04). Retrieved on 2011-11-22.
- ↑ Kinn, John J "Chemiluminescent kite" U.S. Patent 4,715,564issued 12/29/1987
- ↑ Venkata Nori and Jerry Seitzman - AIAA - 2008
| ||||||||||||||||||||||||||||||||||||||||||||||