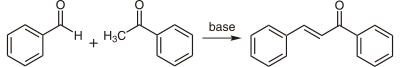Chalcone
- For the genus of grass skipper butterflies, see Chalcone (butterfly).
| Chalcone[1] | |
|---|---|
 | |
| IUPAC name 1,3-Diphenyl-2-propen-1-one | |
| Other names Chalcone | |
| Identifiers | |
| CAS number | 94-41-7 614-47-1 ((E)-Chalcone) |
| PubChem | 637760 |
| ChemSpider | 6921 |
| ChEBI | CHEBI:27618 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C15H12O |
| Molar mass | 208.26 g mol−1 |
| Density | 1.071 g/cm3 |
| Melting point | 55–57 °C |
| Boiling point | 345–348 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Chalcone is an aromatic ketone and an enone that forms the central core for a variety of important biological compounds, which are known collectively as chalcones or chalconoids. Benzylideneacetophenone is the parent member of the chalcone series. The alternative name given to chalcone are phenyl styryl ketone, benzalacetophenone, β-phenylacrylophenone, ɣ-oxo-α,ɣ-diphenyl-α-propylene and α-phenyl-β-benzoylethylene.
Chemical properties
Chalcones have two absorption maxima at 280 nm and 340 nm.[2]
Chemical reactions
Synthesis
Chalcones can be prepared by an aldol condensation between benzaldehyde and acetophenone in the presence of sodium hydroxide as a catalyst.
This reaction has been found to work without any solvent at all - a solid-state reaction.[3] The reaction between substituted benzaldehydes and acetophenones has been used to demonstrate green chemistry in undergraduate chemistry education.[4] In a study investigating green chemistry synthesis, chalcones were also synthesized from the same starting materials in high temperature water (200 to 350 °C).[5]
Alternatively, the substituted chalcones were synthesised by piperidine mediated condensation to avoid side reactions such as multiple condensations,polymerizations, and rearrangements.[6]
other reactions
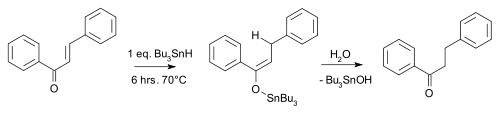
An example is the carbonyl reduction of chalcone by tributyltin hydride:[7]
An enantioselective version of this reaction has also been developed.[8]
See also
References
- ↑ Merck Index, 11th Edition, 2028.
- ↑ Photochemistry of chalcone and the application of chalcone-derivatives in photo-alignment layer of liquid crystal display. Dong-mee Song, Kyoung-hoon Jung, Ji-hye Moon and Dong-myung Shin, Optical Materials, 2002, volume 21, pages 667–671, doi:10.1016/S0925-3467(02)00220-3
- ↑ Toda, F., et al., J. Chem. Soc. Perkin Trans. I, 1990, 3207.
- ↑ Palleros, D. R., J. Chem. Educ., 81, 1345 (2004).
- ↑ Comisar, C. M. and Savage, P. E. Green Chem., 6 (2004), 227 - 231. doi:10.1039/b314622g
- ↑ P Venkatesan and S Sumathi, "Piperidine Mediated Synthesis of N-Heterocyclic Chalcones and Their Antibacterial Activity", J. Heterocyclic Chem., 47, 81 (2010).
- ↑ Leusink, A.J.; Noltes, J.G. (1966). "Reaction of organotin hydrides with α,β-unsaturated ketones". Tetrahedron Letters 7 (20): 2221. doi:10.1016/S0040-4039(00)72405-1.
- ↑ Moritani, Yasunori; Appella, Daniel H.; Jurkauskas, Valdas; Buchwald, Stephen L. (2000). "Synthesis of β-Alkyl Cyclopentanones in High Enantiomeric Excess via Copper-Catalyzed Asymmetric Conjugate Reduction". Journal of the American Chemical Society 122 (28): 6797. doi:10.1021/ja0009525.