Cerberin
| Cerberin | ||
|---|---|---|
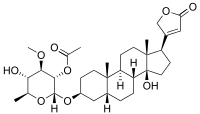 | ||
| IUPAC name [(2R,3S,4R,5S,6S)-5-Hydroxy-2-[[(3S,5R,8R,9S,10S,13R,14S,17R)-14-hydroxy-10,13-dimethyl-17-(5-oxo-2H-furan-3-yl)-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-3-yl]oxy]-4-methoxy-6-methyloxan-3-yl] acetate | ||
| Other names 2'-Acetylneriifolin | ||
| Identifiers | ||
| CAS number | 25633-33-4 | |
| PubChem | 10031063 | |
| ChEBI | CHEBI:75049 | |
| Jmol-3D images | Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C32H48O9 | |
| Molar mass | 576.72 g mol−1 | |
| Melting point | 191 °C (376 °F)[1] | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Cerberin is a part of the cardiac glycosides group, also known as digitalis-like compounds, which is a group that is used as a treatment for heart problems.[2] In higher doses however these compounds are toxic. Cerberin is a compound which is found in Cerbera odollam (also known as the suicide tree). In previous centuries, about 3,000 people per year were killed because they were poisoned by this tree, and nowadays this tree causes 50% of the plant-poison cases and 10% of the total poisonings.[3]
0.08 till 0.16% of the seeds of this tree contain cerberin, the seeds are found in the kernels of the fruits.[1] Cerberin is absorbed by the body via the skin, when handling the kernels, or through the gastrointestinal tract when it is eaten. This tree can be used for homicide when the kernels are mixed with food and the taste can even be masked with strong spices. For suicide, people simply consume the kernels in any way, mostly they mash the kernel with jaggery (gur) and eat it as a sweet.[3]
Structure and reactivity
Cerberin is soluble in chloroform, aceton and moderately in water.[1] All the cardiac glycosides have the same structural motif, a steroid nucleus. These four rings are the pharmacophoric moiety and are responsible for the reactivity of cardiac glycosides. The steroid core is connected with a lactone ring and a sugar part (see figure 1 in Prassas[4]).There are two types of cardiac glycosides depending on de characteristics of the lactone moiety. Because cerberin has a five-membered ring it belongs to the cardenolides (see figure 1 and figure 1 in Prassas[4]). Many types of sugars can be attached as the sugar moiety of the cardiac glycosides such as glucose, galactose and mannose. What kind of sugar is attached plays a role in the pharmacodynamic and pharmacokinetic phase in the body. Some sugars can be faster metabolized than others, so the sugar determines the power of the cardiac glycoside.[4]
Metabolism
Since there have not been any excessive studies on cerberin, very little is known about the metabolism of this compound. The structure of cerberin is a lot like digoxin, so the estimation is that cerberin is eliminated in the same way as digoxin. For digoxin the largest part (60-80%) is excreted unchanged by the kidneys, the other part is mostly metabolised by the liver.[5] During the metabolism of cerberin a mixture of 2 types of reactions could occur. The first reaction is called a phase 1 reaction, it basically modifies the structure of the compound. The phase 1 reaction gives the ability to put a functional group on the compound in the second reaction. The second reaction is called a phase 2 conjugation reaction and adds a large polar group to the compound to make it less lipophilic to make it easier for the body to excrete the compound. The phase 1 reaction does not necessarily have to happen for the phase 2 reaction to occur, since there are already some hydroxyl groups present in the structure of cerberin. If the first reaction occurs, any of the methyl groups could be oxidized, forming a hydroxymethyl group. During the second reaction a functional group reacts with the hydroxyl to add the functional group to the compound. The functional group will most likely be either glutathione or glucuronide, which are used the most by the body to detoxify compounds. The half-life for digoxin, also a cardiac glycoside with a similar structure is 36–48 hours for people with a normal renal function and up to 6 days for people with a compromised renal function. This makes the renal function an important factor in the toxicity of digoxin and thus for cerberin as well.[6]
Mechanism of action
Cerberin binds to and inhibits the cellular Na+/K+ -ATPase, because it binds to the alpha-subunit of the enzyme. This is the catalytic moiety. There are also a beta- and FXYD subunits. These two subunits influence the affinity of cerberin to Na+/K+ -ATPase. The expression of the beta- and FXYD-subunit is tissue-specific. Because of this, cerberin will have different effects in different tissues. When cerberin binds to the Na+/K+-ATPase the conformation of the enzyme changes. This will lead to the activation of signal transduction pathways in the cell.[4] A detailed description of the effects of cerberin in the cell is given below.
Na+/K+-ATPase pump
Na+/K+-ATPase is an ion transport system of sodium and potassium ions and requires energy. It is often used in many types of cellular systems. Sodium ions move out of the cell and potassium ions enter the cell (3:2) with the aid of this pump. During the transport of these ions, the enzyme undergoes several changes in conformation. Including a phosphorylation and dephosphorylation step.[7] The transport of Na+ and K+ is important for cell survival. Cardiac glycosides, such as cerberin, alter the transport of ions against their gradient. Cerberin is able to bind to the extracellular part of the Na+/K+-ATPase pump and can block the dephosphorylation step. Due to this inhibition it is impossible to transport sodium and potassium across the membrane and results in raising intracellular concentration of Na+.
Na+/Ca2+-exchanger
Accumulation of intracellular sodium ions cause an increase of intracellular calcium. This is because the calcium-sodium exchange pump’s activity decreases. The calcium-sodium exchange pump exchanges Ca2+ and Na+ without the use of energy.[8] This exchanger is essential for maintaining sodium and calcium homeostasis. The exact mechanism by which this exchanger works is unclear. It is known that calcium and sodium can move in either direction across the membrane of muscle cells. It is also known that three sodium ions are exchanged for each calcium and that an increase in intracellular sodium concentration through this exchange mechanism leads to an increase in intracellular calcium concentration. As intracellular sodium increases, the concentration gradient driving sodium into the cell across the exchanger is reduced. As a result, the activity of the exchanger is reduced, which decreases the movement of calcium out of the cell.[2]
Thus by inhibiting the Na+/K+-ATPase, cardiac glycosides cause intracellular sodium concentration to increase. This leads to an accumulation of intracellular calcium via the Na+/Ca2+-exchange system with the following effects:
- In the heart, increased intracellular calcium causes more calcium to be released, thereby making more calcium available to bind to troponin-C, which increases contractility (inotropy).
- Inhibition of the Na+/K+-ATPase in vascular smooth muscle causes depolarization, which causes smooth muscle contraction.[2]
The conformational change of Na+/K+-ATPase plays not only a role in the contraction of muscles, but also in cell growth, cell motility and apoptosis. Due to de binding of cerberin, specific second messengers can be activated. After a cascade of cellular interactions nuclear transcription factors binds to the DNA and new enzymes will be made. This enzymes can for example play a role in cell proliferation.[4]
Efficacy and side effects
There is still little known about the adverse effects of cerberin itself. Just that the effects that occur in digitalis will probably also occur in other cardiac glycosides such as cerberin. It reduces the heart rate which can be partially abolished by atropine, but only when given in low doses. With higher doses (0.5 to 1 kernel is enough) the poisons effects cannot be reduced by atropine anymore and the patient dies. But there is no direct correlation between the dose and the mortality.[3]
Within an hour of intake of the poison/consumption of the white kernel – which is taken out of the seed by removing its green skin and then mashed – there can occur retching, nausea, vomiting and abdominal pain.[3][9] Also the patient becomes drowsy and weak and thus may get into coma. Arrhythmia will be observed, the heartbeat will be too slow (bradycardia).[9] The patient will not respond to atropine anymore, and also hyperkalemia was produced by the poisoning. The greater the toxicity, the higher the potassium concentration in the plasma. After three to six hours, death will likely occur.[3]
The pharmacological actions of cerberin result in electrocardiogram (ECG) changes. Such as various types of bradycardia like sinus bradycardia, AV dissociation and junctional rhythms.[9] Second-degree Sinoatrial block and nodal rhythm were described.[3] There can also be ST depression or T wave inversion, these do not indicate toxicity. However, PR interval prolongation may be a sign of (cerberin) toxicity.[10]
Therapeutic uses
Digitalis compounds are widely used for many years in the treatment of chronic heart failure and arrhythmias. New research has found that cardiac glycosides can be useful in cancer therapies.
Heart failure
Although newer and more efficacious treatments for heart failure are available, digitalis compounds are still used.[2] Because of the inhibition of the Na+/K+-ATPase-pump, the amount of sodium ions in cardiac muscle cells raises, which leads to an increased level of calcium ions (see mechanism of action) and an increase in cardiac contractile force (see mechanism of action).[4]
Arrhythmias
Cardiac glycosides can reduce the ventricular rate because of the ability to activate vagal efferent nerves to the heart. Vagal activation leads to blocking of the electrical impulses. When this occurs, fewer impulses reach the ventricles and ventricular rate falls. This is also called the parasympathomimetic effect. Digitalis-like compounds also increase the refractory period. Because of this it takes longer to create new electrical impulses, therefore the ventricular rate decreases.[2]
Cancer therapy
Research has found that the intake of cardiac glycosides can reduce the likelihood to get cancer, because of the antiproliferative and apoptotic effects. The exact mechanism of these effects is not yet known.[4]
Toxicity
The response of cerberin depends on the tissue, exposure time and the dose.[4] The cardiac toxins act mainly on the heart, either directly or through the nerves. They also have a relatively long half-life. Because cerberin is so similar to other cardiac glycosides, it is likely that the half-life of cerberin will be in the same magnitude. Ouabain for example has a half-life of 20 hours and digoxin has a half-life of 40 hours. Due to this it will take a few days before the steady state is reached with a constant dosage. This is approximately 4 to 5 times the half-life. The cerberin concentration at steady state is called the therapeutic plasma concentration. For digoxin this value lies between 0.5 and 1.5 ng/mL. When this margin is exceeded, the dose is toxic and can even be life-threatening. Because of the long half-life it could take days before the plasma concentration is decreased to a safe value.[2] The therapeutic index of cardiac glycosides, like cerberin, is about a ratio of 2:1.[11] This is quite a narrow index which indicates that cerberin is a pretty toxic compound (A small dose is needed for the compound to be toxic), compared to for example cocaine which already is a ‘less-safe’ drug.[11]
The lethal dose of cerberin for a dog is 1.8, for a cat 3.1 and for a rabbit 50 in units of mg per kilo bodyweight.[1] Eating the core of the fruit from the suicide tree is already enough to receive a lethal dose. The period in where the toxin becomes fatal lasts approximately 1 to 2 days. However, potassium supplements can be used to counter the toxic effects of cerberin.[2] Another way to get rid of the toxin is to have gastric lavage. Symptoms of intoxication are, but not limited to: burning sensations in the mouth, vomiting, diarrhea, headache, dilated pupils, irregular beating of the heart followed by drowsiness, coma and eventually death.[12]
References
- ↑ 1.0 1.1 1.2 1.3 Chopra, R. N., & Chopra, I. C. (2006). Chopra's indigenous drugs of india.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Klabunde, R. E. "cardiostimulatory". Retrieved Retrieved 03 16, 2013.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Gaillarda, Y., Krishnamoorthyb, A., & Bevalot, F. (12). "Cerbera odollam: a 'suicide tree' and cause of death in the state of Kerala, India". Journal of Ethnopharmacology 95 (2–3): 123–126. doi:10.1016/j.jep.2004.08.004. PMID 15507323.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Prassas, I., & Diamandis, E. P. (2008). "Novel of therapeutic applications of cardiac glycosides". Nature reviews 7 (11): 926–930. doi:10.1038/nrd2682. PMID 18948999.
- ↑ "Digoxin lecture". Retrieved Retrieved 03 13, 2013.
- ↑ Timbrell, J. A. (2009). Princples of biochemical toxicoly.
- ↑ Godfraind, T. (1984). "Mechanism of action of cardiac glycosides". European heart Journal. 5 Suppl F: 303–8. PMID 6099806.
- ↑ Fozzard, H. A., & Sheets, M. F. (1985). "Cellular mechanism of action of cardiac glycosides". Journal of the American College of Cardiology 5 (5 Suppl A): 10A–15A. PMID 2580874.
- ↑ 9.0 9.1 9.2 Makama, F. (7 February 2011). "The Negative Effect of Cerbera Odollam as a Common Poison" (Cerbera odollam). ezinearticles.com. Retrieved 29 April 2013.
- ↑ Doering W, König E, Sturm W (1977). "(title in German)" [Digitalis intoxication: specifity and significance of cardiac and extracardiac symptoms. part I: Patients with digitalis-induced arrhythmias (author's transl)]. Zeitschrift für Kardiologie (in German) 66 (3): 121–128. PMID 857452.
- ↑ 11.0 11.1 Becker, D.E. (2007). "Drug therapy in dental practice: general principles. Part 2 - pharmacodynamic considerations.". Anesthesia Progress 54 (1): 19–24. doi:10.2344/0003-3006(2007)54[19:DTIDPG]2.0.CO;2. PMC 1821133. PMID 17352523.
- ↑ Rao, N. G. (2000). Textbook of forensic medicine and toxicology.