Centaureidin
From Wikipedia, the free encyclopedia
| Centaureidin | |
|---|---|
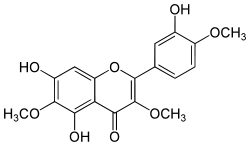 | |
| IUPAC name 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,6-dimethoxychromen-4-one | |
| Other names Desmethoxycentaureidine | |
| Identifiers | |
| CAS number | 17313-52-9 |
| PubChem | 5315773 |
| ChemSpider | 4474997 |
| ChEBI | CHEBI:69356 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C18H16O8 |
| Molar mass | 360.31 g/mol |
| Density | 1.542 g/mL |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Centaureidin is an O-methylated flavonol. It can be isolated from Tanacetum microphyllum,[1] Brickellia veronicaefolia, Bidens pilosa[2] and Polymnia fruticosa.[3]
References
- ↑ Abad, Maria Jose; Bermejo, Paulina; Villar, Angel (1995). "The activity of flavonoids extracted from Tanacetum microphyllum DC. (Compositae) on soybean lipoxygenase and prostaglandin synthetase". General Pharmacology: the Vascular System 26 (4): 815–9. doi:10.1016/0306-3623(94)00242-F. PMID 7635257.
- ↑ Chang, Shu-Lin; Chiang, Yi-Ming; Chang, Cicero Lee-Tian; Yeh, Hsu-Hua; Shyur, Lie-Fen; Kuo, Yueh-Hsiung; Wu, Tung-Kung; Yang, Wen-Chin (2007). "Flavonoids, centaurein and centaureidin, from Bidens pilosa, stimulate IFN-γ expression". Journal of Ethnopharmacology 112 (2): 232–6. doi:10.1016/j.jep.2007.03.001. PMID 17408892.
- ↑ Beutler, John A.; Cardellina, John H.; Lin, Chii M.; Hamel, Ernest; Cragg, Gordon M.; Boyd, Michael R. (1993). "Centaureidin, a cytotoxic flavone from Polymnia fruticosa, inhibits tubulin polymerization". Bioorganic & Medicinal Chemistry Letters 3 (4): 581–4. doi:10.1016/S0960-894X(01)81233-6.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.