Ceftibuten
 | |
|---|---|
| Systematic (IUPAC) name | |
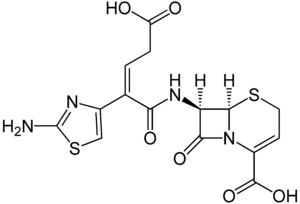
| (6R,7R)-7-([(Z)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino) -8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Clinical data | |
| Trade names | Cedax |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a698023 |
| Legal status | ? |
| Identifiers | |
| CAS number | 97519-39-6 |
| ATC code | J01DD14 |
| PubChem | CID 5282242 |
| DrugBank | DB01415 |
| ChemSpider | 4445419 |
| UNII | IW71N46B4Y |
| KEGG | D00922 |
| ChEBI | CHEBI:3510 |
| ChEMBL | CHEMBL1605 |
| Chemical data | |
| Formula | C15H14N4O6S2 |
| Mol. mass | 410.427 g.mol-1 |
| SMILES
| |
| |
| | |
Ceftibuten is a third-generation cephalosporin antibiotic. It is an orally-administered agent, with 2 dosage forms, capsule or oral suspension. It is marketed by Pernix Therapeutics under the trade name Cedax.
It is active against Haemophilus influenzae, Moraxella catarrhalis, Escherichia coli (K. pneumoniae, K. oxytoca), Proteus vulgaris, P. mirabils, P. providence, Salmonella sp., Shigella sp., Enterobacter sp. and Streptococcus sp.
Clinical use
Indications
Ceftibuten is used to treat acute bacterial exacerbations of chronic bronchitis (ABECB), acute bacterial otitis media, pharyngitis, and tonsilitis. It is also indicated for pneumonia, infections of the urinary tract, enteritis and gastroenteritis.[citation needed]
Adverse reactions
In studies made in 3,000 patients ceftibuten was well tolerated. Most frequent reactions were gastrointestinal and nauseas.
Formulations
Ceftibuten is available as capsules containing 400 mg, and a powder for oral suspension containing 180 mg per 5 ml.
External links
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||