Catechin-7-O-glucoside
| Catechin-7-O-glucoside | ||
|---|---|---|
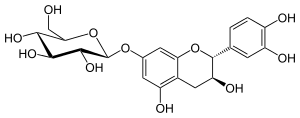 | ||
| IUPAC name (2S,4S,5S)-2-[[(2R,3S)-2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-3,4-dihydro-2H-chromen-7-yl]oxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| Other names (2R,3S)-Catechin-7-O-β-D-glucopyranoside | ||
| Identifiers | ||
| PubChem | 44257085 | |
| Jmol-3D images | Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C21H24O11 | |
| Molar mass | 452.41 g mol−1 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Catechin-7-O-glucoside is a flavan-3-ol glycoside formed from catechin.
Natural occurrences
Catechin-7-O-glucoside can be found in Bergenia ciliata[1] or can be isolated from the hemolymph of the European pine sawfly (Neodiprion sertifer).[2]
It can also be produced by biotransformation of (+)-catechin by cultured cells of Eucalyptus perriniana.[3]
Presence in natural traditional drugs
Catechin-7-O-glucoside can be found in Paeoniae Radix, the crude drug made from roots of the Chinese peony (Paeonia lactiflora),[4] in the red knotweed (Bistorta macrophylla, also known as Polygonum macrophyllum),[5] in the stem barks of the Nepali hog plum (Choerospondias axillaris),[6] in the Korean plum yew (Cephalotaxus koreana)[7] and in Huanarpo Macho (Jatropha macrantha).[8]
Presence in food
It is found in buckwheat groats,[9] in the red bean (the seed of Vigna umbellata, formerly known as Phaseolus calcaratus),[10] in barley (Hordeum vulgare L.) and malt.[11]
Health effects
This compound has an antioxidant activity leading to a cytoprotective effect.[10][12]
References
- ↑ Dharmender, Rathee; Madhavi, Thanki; Reena, Agrawal; Sheetal, Anandjiwala (2010). "Simultaneous Quantification of Bergenin, (+)-Catechin, Gallicin and Gallic acid; and Quantification of β-Sitosterol using HPTLC from Bergenia ciliata (Haw.) Sternb. Forma ligulata Yeo (Pasanbheda)". Pharmaceutica Analytica Acta 01. doi:10.4172/2153-2435.1000104.
- ↑ Vihakas, Matti; Tähtinen, Petri; Ossipov, Vladimir; Salminen, Juha-Pekka (2012). "Flavonoid Metabolites in the Hemolymph of European Pine Sawfly (Neodiprion sertifer) Larvae". Journal of Chemical Ecology 38 (5): 538–46. doi:10.1007/s10886-012-0113-y. PMID 22527054.
- ↑ Biotransformation of (+)-catechin by plant cultured cells of Eucalyptus perriniana. Otani S, Kondo Y, Asada Y, Furuya, Hamada, Nakajima, Ishihara and Hamada H, Plant Biotechnol., 2004, Vol. 21, No. 5, pages 407-409 (abstract)
- ↑ New Monoterpene Glycoside Esters and Phenolic Constituents of Paeoniae Radix, and Increase of Water Solubility of Proanthocyanidins in the Presence of Paeoniflorin. Takashi Tanaka, Maki Kataoka, Nagisa Tsuboi and Isao Kouno, Chem. Pharm. Bull., 2000, 48(2), pages 201—207
- ↑ Wang, S; Wang, D; Feng, S (2004). "Studies on chemical constituents from Polygonum macrophyllum". Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials 27 (6): 411–3. PMID 15524292.
- ↑ Flavanoidal constituents of Choerospondias axillaries and their in vitro antitumor and anti-hypoxia activities. Li Chang-wei, Cui Cheng-bin, Cai Bing, Han Bing, Li Ming-ming and Fan Ming, Chinese Journal Of Medicinal Chemistry, 2009, 19 (1), pages 48-51,64 (abstract)
- ↑ Yoon, Kee Dong; Jeong, Doc Gyun; Hwang, Yun Ha; Ryu, Jei Man; Kim, Jinwoong (2007). "Inhibitors of Osteoclast Differentiation fromCephalotaxus koreana". Journal of Natural Products 70 (12): 2029–32. doi:10.1021/np070327e. PMID 17994703.
- ↑ Benavides, Angelyne; Montoro, Paola; Bassarello, Carla; Piacente, Sonia; Pizza, Cosimo (2006). "Catechin derivatives in Jatropha macrantha stems: Characterisation and LC/ESI/MS/MS quali–quantitative analysis". Journal of Pharmaceutical and Biomedical Analysis 40 (3): 639–47. doi:10.1016/j.jpba.2005.10.004. PMID 16300918.
- ↑ Report on cereals at Phenol-Explorer.eu. Retrieved 18 December 2012.
- ↑ 10.0 10.1 Baek, Jin-A; Son, Young-Ok; Fang, Minghao; Lee, Young Jae; Cho, Hyoung-Kwon; Whang, Wan Kyunn; Lee, Jeong-Chae (2011). "Catechin-7-O-β-d-glucopyranoside scavenges free radicals and protects human B lymphoma BJAB cells on H2O2-mediated oxidative stress". Food Science and Biotechnology 20: 151. doi:10.1007/s10068-011-0021-x., INIST:23809899
- ↑ Friedrich, Wolfgang; Galensa, Rudolf (2002). "Identification of a new flavanol glucoside from barley ( Hordeum vulgare L.) and malt". European Food Research and Technology 214 (5): 388. doi:10.1007/s00217-002-0498-x.
- ↑ Kim, Ki Cheon; Kim, Jin Sook; Ah Kang, Kyoung; Kim, Jong Min; Won Hyun, Jin (2010). "Cytoprotective effects of catechin 7-O-β-D glucopyranoside against mitochondrial dysfunction damaged by streptozotocin in RINm5F cells". Cell Biochemistry and Function 28 (8): 651–60. doi:10.1002/cbf.1703. PMID 21104932.
| |||||||||||||||||