Castleman's disease
| Castleman's disease | |
|---|---|
| Classification and external resources | |
 Micrograph of Castleman's disease, hyaline vascular variant, exhibiting the characteristically expanded mantle zone and a radially penetrating sclerotic blood vessel ("lollipop" sign). H&E stain. | |
| ICD-9 | 785.6 |
| DiseasesDB | 2165 |
| MeSH | D005871 |
Castleman's disease (giant or angiofollicular lymph node hyperplasia, lymphoid hamartoma, angiofollicular lymph node hyperplasia) is an uncommon lymphoproliferative disorder that may be localized to a single lymph node (unicentric) or occur systemically (multicentric). It must be distinguished from reactive lymph node hyperplasia and malignancies.[1] It is a very rare disorder characterized by non-cancerous growths (tumors) that may develop in the lymph node tissue at a single site or throughout the body.[2] It involves hyperproliferation of certain B cells that often produce cytokines. While not officially considered a cancer, the overgrowth of lymphocytes with this disease is similar to lymphoma.[3]
It is named after Benjamin Castleman.[4][5]
Types

There are several variants of Castleman's disease.
In most of the cases, Castleman's disease is likely due to hypersecretion of the cytokine IL-6,[6] but some patients may have normal IL-6 levels and present with non-iron-deficient microcytic anemia.[1]
- In tumors that are positive for Kaposi's sarcoma-associated herpesvirus (KSHV), also called human herpes virus 8 (HHV-8), this is most likely due to expression of the virus-encoded cytokine, vIL-6.[7]
- KSHV negative tumors appear to be the result of over-secretion of human IL-6.
Unicentric vs. multicentric
Unicentric Castleman's disease involves tissue growths at only a single site. It usually has few or no symptoms other than those directly associated with the l enlargement of the lymph node. In 90% or more, removal of the enlarged node is curative, with no further complications. However, in 2011, Weng et al. described a patient with unicentric Castleman's disease, hyaline vascular type, presenting with severe chronic non-iron-deficient anemia. He suggested that in patients with normal IL-6 level may present with non-iron deficient type and may resolve after effective treatment of Castleman's disease.[1]
Multicentric Castleman's disease (MCD) involves growths at multiple sites.[8] About 50% is caused by KSHV, also called HHV-8, a gammaherpesvirus that is also the cause of Kaposi's sarcoma and primary effusion lymphoma, while the remainder of MCD are of unknown cause. The form of MCD most closely associated with KSHV is the plasmacytic form of Castleman's disease while another pathologic form, the hyaline-vascular form, is generally negative for this virus.
MCD Symptoms
The most common 'B Symptoms' of MCD are high fevers, anemia, weight loss, loss of appetite, and low white blood cell counts, which may to be due to the overproduction of interleukin 6. Symptomatically, therefore, MCD can be difficult to diagnose and even in the case of a lymph-node biopsy a conclusive diagnosis remains problematic.
Castleman's is seen in POEMS syndrome and is implicated in 10% of cases of paraneoplastic pemphigus.
Treatment
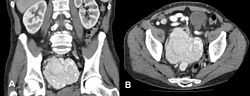
Unicentric
In the unicentric form of the disease, surgical resection is often curative,[1][9] and the prognosis is excellent.
Multicentric
There is no standard therapy for multicentric Castleman's disease. Treatment modalities include antiviral drugs such as ganciclovir for human herpesvirus type 8 (HHV-8), chemotherapy, corticosteroids, immunomodulators, monoclonal antibodies against IL-6, and thalidomide.
It is important to distinguish AIDS-related multicentric Castleman’s disease from other forms of multicentric Castleman’s disease. Treatment for the former can be focused upon the same protocols used for treating the underlying AIDS.[10]
Prior to 1996 MCD carried a poor prognosis of about 2 years, due to autoimmune hemolytic anemia and non-Hodgkin's lymphoma which may arise as a result of proliferation of infected cells. The timing of diagnosis, with particular attention to the difficulty of determining the cause of B symptoms without a CT scan and lymph node biopsy, may impact significantly on the prognosis and risk of death. Left untreated, MCD usually gets worse and becomes increasingly difficult and unresponsive to current treatment regimens.
Recent work with HIV-positive patients with KSHV-related MCD suggests that treatment with the antiherpesvirus drug ganciclovir or the antiCD20 B cell monoclonal antibody, rituximab, may markedly improve outcome. These drugs target and kill B cells via the B cell specific CD20 marker. Since B cells are required for the production of antibodies, the body's immune response is weakened whilst on treatment and the risk of further viral or bacterial infection is increased. Due to the uncommon nature of the condition there are not many large scale research studies from which standardized approaches to therapy may be drawn, and the extant case studies of individuals or small cohorts should be read with caution. As with many diseases, the patient's age, physical state and previous medical history with respect to infections may impact the disease progression and outcome.
Use of tocilizumab has been proposed.[11]
Siltuximab, a monoclonal antibody that binds interleukin-6, preventing it from binding to the IL-6 receptor, was submitted to the U.S. Food and Drug Administration for approval in October, 2013.[12] [13] Preliminary data suggest that treatment siltuximab may achieve tumour and symptomatic response in 34% of patients with MCD.[14]
Other treatments for multicentric castleman disease include the following:
- Corticosteroids
- Chemotherapy
- Thalidomide[15]
See also
References
- ↑ 1.0 1.1 1.2 1.3 Weng, Chien-Hsiang; Joe-Bin Chen, John Wang, Cheng-Chung Wu, Yuan Yu, Tseng-Hsi Lin (23 August 2011). "Surgically Curable Non-Iron Deficiency Microcytic Anemia: Castleman’s Disease". Onkologie 34 (8-9): 456–458. doi:10.1159/000331283. PMID 21934347.
- ↑ Bucher P, Chassot G, Zufferey G, Ris F, Huber O, Morel P (June 2005). "Surgical management of abdominal and retroperitoneal Castleman's disease". World J Surg Oncol 3: 33. doi:10.1186/1477-7819-3-33. PMC 1166581. PMID 15941478.
- ↑ "Castleman disease". Mayo Clinic. Retrieved 2010-02-03.
- ↑ synd/3017 at Who Named It?
- ↑ Castleman B, Iverson L, Menendez VP (1956). "Localized mediastinal lymphnode hyperplasia resembling thymoma". Cancer 9 (4): 822–30. doi:10.1002/1097-0142(195607/08)9:4<822::AID-CNCR2820090430>3.0.CO;2-4. PMID 13356266.
- ↑ Ahmed B, Tschen JA, Cohen PR, et al. (September 2007). "Cutaneous castleman's disease responds to anti interleukin-6 treatment". Mol. Cancer Ther. 6 (9): 2386–90. doi:10.1158/1535-7163.MCT-07-0256. PMID 17766835.
- ↑ Aoki Y, Yarchoan R, Wyvill K, Okamoto S, Little RF, Tosato G (April 2001). "Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders". Blood 97 (7): 2173–6. doi:10.1182/blood.V97.7.2173. PMID 11264189.
- ↑ Menezes BF, Morgan R, Azad M (2007). "Multicentric Castleman's disease: a case report". J Med Case Reports 1: 78. doi:10.1186/1752-1947-1-78. PMC 2014764. PMID 17803812.
- ↑ Talarico F, Negri L, Iusco D, Corazza GG (April 2008). "Unicentric Castleman's disease in peripancreatic tissue: case report and review of the literature". G Chir 29 (4): 141–4. PMID 18419976.
- ↑ Sprinz E, Jeffman M, Liedke P, Putten A, Schwartsmann G (February 2004). "Successful treatment of AIDS-related Castleman's disease following the administration of highly active antiretroviral therapy (HAART)". Ann. Oncol. 15 (2): 356–8. doi:10.1093/annonc/mdh066. PMID 14760135.
- ↑ Matsuyama M, Suzuki T, Tsuboi H, et al. (2007). "Anti-interleukin-6 receptor antibody (tocilizumab) treatment of multicentric Castleman's disease" (– Scholar search). Intern. Med. 46 (11): 771–4. doi:10.2169/internalmedicine.46.6262. PMID 17541233.
- ↑ "A Study to Evaluate the Safety of Long-term Treatment With Siltuximab in Patients With Multicentric Castleman's Disease". ClinalTrails.gov.
- ↑ "First IL-6–blocking drug nears approval for rare blood disorder". Nature Medicine. October 7, 2013.
- ↑ Wong, Raymond; Corey Casper, Nikhil Munshi, Xiaoyan Ke, Alexander Fosså, David Simpson, Marcelo Capra, Ting Liu, MD, Ruey Kuen Hsieh, Yeow Tee Goh, Jun Zhu, Seok-Goo Cho, Hanyun Ren, James Cavet, Rajesh Bandekar, Margaret Rothman, Thomas A Puchalski, Shalini Chaturvedi, Helgi van de Velde, Jessica Vermeulen and Frits van Rhee (9 December 2013). "A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study Of The Efficacy and Safety Of Siltuximab, An Anti-Interleukin-6 Monoclonal Antibody, In Patients With Multicentric Castleman’s Disease". American Society of Hematology 55th Annual Meeting. Retrieved 13 December 2013.
- ↑ http://www.mayoclinic.com/health/castleman-disease/DS01000/DSECTION=treatments-and-drugs
External links
| |||||||||||||||||||||||