Carboxylate


A carboxylate is a salt or ester of a carboxylic acid. Carboxylate salts have the general formula M(RCOO)n, where M is a metal and n is 1,2,...; carboxylate esters have the general formula RCOOR'. R and R' are organic groups; R'≠H.
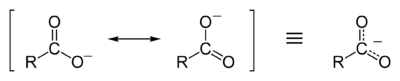
A carboxylate ion is the conjugate base of a carboxylic acid, RCOO−. It is an ion with negative charge.
Resonance stabilization of the carboxylate ion
Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between the two electronegative oxygen atoms in a resonance structure.
This delocalization of the electron cloud means that either of the oxygen atoms is less strongly negatively charged; the positively charged proton is therefore less strongly attracted back to the carboxylate group once it has left. In contrast, an alkoxide ion, once formed, would have a strong negative charge on the oxygen atom, which would make it difficult for the proton to escape. Thus, the carboxylate ion is more stable and carboxylic acids have a lower pH than alcohols: the higher the number of protons in solution, the lower the pH.[1]
Uses
Polycarboxylate ethers serve as the main component of superplasticizers, admixtures used in the construction industry.
Examples
- Formate ion, HCOO−
- Acetate ion, CH3COO−
- Lactate ion, CH3CH(OH)COO−
- Oxalate ion, (COO)22−
- Citrate ion, C3H5O(COO)33−
References