Carbonless copy paper
Carbonless copy paper (CCP), non-carbon copy paper, or NCR paper is an alternative to carbon paper, used to make a copy of an original, handwritten (or mechanically typed) document without the use of any electronics. The process was invented in 1952 by chemists Lowell Schleicher and Barry Green, working for the NCR Corporation, as a biodegradable, stain-free alternative to carbon paper.[1] Early product literature piggybacked on NCR's corporate name by calling the paper No Carbon Required paper, a bacronym of National Cash Register.
Operation
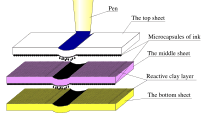
Carbonless copy paper consists of sheets of paper that are coated with micro-encapsulated dye or ink and/or a reactive clay. The back of the first sheet is coated with micro-encapsulated dye. The lowermost sheet is coated on the top surface with a clay that quickly reacts with the dye to form a permanent mark. Any intermediate sheets are coated with clay on top and dye on the bottom.
When someone writes on the sheets, the pressure from the point of the writing instrument causes the micro-capsules to break and spill their dye. Since the capsules are so small, the print obtained is very accurate. Similarly, the paper can be used in dot-matrix and impact printers, where the striking head releases dye to interact with the clay.
Carbonless copy paper was also available in a self-contained version that had both the ink and the clay on the same side of the paper.
Uses
Carbonless copy paper, commonly known in the printing industry as NCR paper, was first produced by the NCR Corporation. Before this the usual options were to write documents more than once or use carbon paper which was inserted between the sheet being written on and the copy; often the user's fingers were stained. The invention of NCR paper made making multiples of hand-written and printed documents easy and clean.
The paper is commonly used for invoices, receipts and any other document where duplication is required. There is an array of colors available for the copy sheet, such as white, yellow, pink, green and blue. In most instances, white is the top copy and subsequent colors are beneath dependent on the number of duplicated pages required. For example, two copies of document being made, the papers would have a white top sheet and one colored second sheet below, which would be called two-part or duplicate. For three copies, there would be a white top part and two colored parts below, called three-part or triplicate. Four copies would be called four-part or quadruplicate. Stacks of ten or more copies were not unknown.
Carbonless copy paper is usually supplied to the end user collated in pads or books which are bound into sets using glue or staples, with loose sets or continuous stationery also being widely used for use in printers.
Dyes and chemicals
The first dye used commercially in this application was crystal violet lactone, which is widely used today. Other dyes and supporting chemicals used are PTSMH (p-toluene sulfinate of Michler's hydrol), TMA (trimellitic anhydride), phenol-formaldehyde resins, azo dyes, DIPN (diisopropyl naphthalenes), formaldehyde isocyanates, hydrocarbon-based solvents, polycyclic aromatic hydrocarbons, polyoxypropylene diamine, epoxy resins, aliphatic isocyanates, Bisphenol A, diethylene triamine, and others. The dyes in carbonless copy papers may cause contact dermatitis in sensitive persons.
Health and environmental concerns
Until the 1970s, when the use of polychlorinated biphenyls (PCBs) was banned due to health and environmental concerns, PCBs were used as a transfer agent in carbonless copy paper.[2][3][4] PCBs are readily transferred to human skin during handling of such papers, and it is difficult to achieve decontamination by ordinary washing with soap and water.[3] In Japan, carbonless copy paper is still treated as a PCB-contaminated waste.[5]
Exposure to certain types of carbonless copy paper or its components has resulted, under some conditions, in mild to moderate symptoms of skin irritation and irritation of the mucosal membranes of the eyes and upper respiratory tract. A 2000 review found no irritation or sensitization on contact with carbonless copy paper produced after 1987.[6] In most cases, good industrial hygiene and work practices should be adequate to reduce or eliminate symptoms. These include adequate ventilation, humidity, and temperature controls; proper housekeeping; minimal hand-to-mouth and hand-to-eye contact; and periodic cleansing of hands.[7]
The University of Florida has found that chronic exposure to carbonless copy paper can be hazardous to a person's health. Scientists there found higher rates of sick leave and illness complaints at the office using large amounts of carbonless copy paper. A study published in Environmental Health Perspectives provides new evidence that exposure to paper dust and carbonless copy paper in office work are related to increased risk of adult-onset asthma.[8]
The average carbonless copy paper contains a high concentration of Bisphenol A, an endocrine disruptor.[9][10][11][12][13]
In 2001, three employees of a medical center in San Francisco filed a lawsuit against their employer, blaming exposure to carbonless copy paper and other chemicals for their inflammatory breast cancer.[14]
See also
- Spirit duplicator AKA Ditto machine
- List of duplicating processes
Notes
- ↑ "Carbonless Paper Pioneer Lowell Schleicher Dies". Appletonideas.com. Retrieved 2013-11-12.
- ↑ de Voogt P, Klamer J C, and Brinkman U A Th (December 1984). "Identification and quantification of polychlorinated biphenyls in paper and paper board using fused silica capillary gas chromatography". Bulletin of Environmental Contamination and Toxicology 32 (1): 45–52. doi:10.1007/BF01607463. PMID 6421348.
- ↑ 3.0 3.1 Kuratsune M and Masuda Y (April 1972). "Polychlorinated Biphenyls in Non-carbon Copy Paper". Environmental Health Perspectives (Environmental Health Perspectives, Vol. 1) 1: 61–62. doi:10.2307/3428153. JSTOR 3428153. PMC 1474878. PMID 17539088.
- ↑ "NPL Site Narrative for Fox River NRDA/PCB Releases". United States Environmental Protection Agency. Retrieved 2009-06-24.
- ↑ "Invitation of proposals concerning to PCB contaminated solid wastes treatment technologies". Hyogo Prefectural Environment Create Center Public Corporation. 2003-01-27. Retrieved 2008-02-20.
- ↑ Graves CG, Matanoski GM, Tardiff RG (2000). "Carbonless copy paper and workplace safety: a review". Regul Toxicol Pharmacol 32 (1): 99–117. doi:10.1006/rtph.2000.1408. PMID 11029273.
- ↑ "National Institute for Occupational Safety and Health - Carbonless Copy Paper". United States National Institute for Occupational Safety and Health. Retrieved 2007-10-13.
- ↑ Jaakkola, Maritta S.; Jouni J.K. Jaakkola (July 2007). "Office Work Exposures and Adult-Onset Asthma". Environmental Health Perspectives (National Institute of Environmental Health Sciences) 115 (7): 1007–1011. doi:10.1289/ehp.9875. PMC 1913573. PMID 17637914.
- ↑ Fukazawa h, H. K.; Hoshino, K.; Shiozawa, T.; Matsushita, H.; Terao, Y. (2001). "Identification and quantification of chlorinated bisphenol a in wastewater from wastepaper recycling plants". Chemosphere 44 (5): 973–979. doi:10.1016/S0045-6535(00)00507-5. PMID 11513431.
- ↑ Raloff, Janet (2009-10-07). "Concerned About BPA: Check Your Receipts". Society for Science and the Public. Retrieved 2009-10-07.
- ↑ Begley, Sharon (Jun 29, 2009). "When Studies Collide". Newsweek. Retrieved 2009-10-09.
- ↑ Stahlhut; Welshons, W.; Swan, S. (2009). "Bisphenol a data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both". Environmental health perspectives 117 (5): 784–789. doi:10.1289/ehp.0800376. PMC 2685842. PMID 19479022.
- ↑ Takemura; Ma, J.; Sayama, K.; Terao, Y.; Zhu, B.; Shimoi, K. (2005). "In vitro and in vivo estrogenic activity of chlorinated derivatives of bisphenol A". Toxicology 207 (2): 215–221. doi:10.1016/j.tox.2004.09.015. PMID 15596252.
- ↑ Lee, Henry K. (December 14, 2001). "Co-workers' rare cancer a mystery". The San Francisco Chronicle. Retrieved 29 November 2009.
References
External links
- Patent: Pressure-sensitive record material
- Hazard Review: Carbonless Copy Paper, from the National Institute for Occupational Safety and Health.
- Scientist Test Carbonless Copy Paper for Sickening Side Effect http://news.ufl.edu/1997/05/22/carbonls/
| |||||||||||||||||
| ||||||||||||||||||||||||||