Carbon nanobud
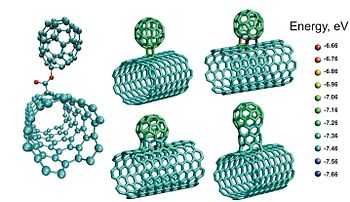
In nanotechnology, carbon nanobuds form a material (discovered and synthesized in 2006) which combines two previously discovered allotropes of carbon: carbon nanotubes and spheroidal fullerenes (or, in short, fullerenes). In this new material, fullerenes are covalently bonded to the outer sidewalls of the underlying nanotube. Consequently, nanobuds exhibit properties of both carbon nanotubes and fullerenes. For instance, the mechanical properties and the electrical conductivity of the nanobuds are similar to those of corresponding carbon nanotubes. However, because of the higher reactivity of the attached fullerene molecules, the hybrid material can be further functionalized through known fullerene chemistry. Additionally, the attached fullerene molecules can be used as molecular anchors to prevent slipping of the nanotubes in various composite materials, thus modifying the composite’s mechanical properties.[1][2]
Due to the large number of highly curved fullerene surfaces acting as electron emission sites on conductive carbon nanotubes, nanobuds possess advantageous field electron emission characteristics. Randomly oriented nanobuds have already been demonstrated to have an extremely low work function for field electron emission. Reported test measurements show (macroscopic) field thresholds of about 0.65 V/μm, (non-functionalized single-walled carbon nanotubes have a (macroscopic) field threshold for field electron emission as high as 2 V/μm) and a much higher current density as compared with that of the corresponding pure single-walled carbon nanotubes.[1] The electron transport properties of certain NanoBud classes have been treated theoretically.[3] The study shows that electrons indeed pass to the neck and bud region of the nanobud system.
Canatu Oy, a Finnish company, claims the intellectual property rights for nanobud material, its synthesis processes, and several applications.[4]
Practical applications
Properties such as chemical reactivity, good dispersion and variable band gap electronic structure suggest wide applicability of nanobuds.[1] As the production processes are scalable, the nanobud applications may have industrial importance. Several theoretical works also mentioned the magnetism in nanobuds.[5][6]
References
- ↑ 1.0 1.1 1.2 Nasibulin, Albert G. et al. (2007). "A novel hybrid carbon material". Nature Nanotechnology 2 (3): 156–161. doi:10.1038/nnano.2007.37. PMID 18654245.
- ↑ Nasibulin, Albert G. et al. (2007). "Investigations of NanoBud formation". Chemical Physics Letters 446: 109–114. doi:10.1016/j.cplett.2007.08.050.
- ↑ Fürst, Joachim A. et al. (2009). "Electronic transport properties of fullerene functionalized carbon nanotubes: Ab initio and tight-binding calculations". Physical Review B 80 (3): 115117. doi:10.1103/PhysRevB.80.035427.
- ↑ "European Patent Office: search CANATU". Retrieved 2010-06-03.
- ↑ "Magnetism in hybrid carbon nanostructures: Nanobuds". Physical Review B 79: 165401. 2009. doi:10.1103/PhysRevB.79.165401.
- ↑ Physical Chemistry Chemical Physics 13: 5945–5951. 2011. doi:10.1039/C0CP02433C.
| |||||||||||||||||||||||