Carbon dioxide scrubber
A carbon dioxide scrubber is a device which absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage.
Technologies
Amine scrubbing
The dominant application for CO2 scrubbing is for removal of CO2 from the exhaust of coal- and gas-fired power plants. Virtually the only technology being seriously evaluated involves the use of various amines, e.g. monoethanolamine. Cold solutions of these organic compounds bind CO2, but the binding is reversed at higher temperatures:
- CO2 + 2HOCH2CH2NH2
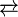 HOCH2CH2NH3+HOCH2CH2NH(CO2-)
HOCH2CH2NH3+HOCH2CH2NH(CO2-)
This technology has only been lightly implemented because of capital costs of installing the facility and the operating costs of utilizing it.[1]
Minerals and zeolites
Several minerals and mineral-like materials reversibly bind CO2.[2] Most often, these minerals are oxides, and often the CO2 is bound as carbonate. Carbon dioxide reacts with quicklime (calcium oxide), to form limestone (calcium carbonate).[3] The process is called Carbonate Looping. Other minerals include serpentinite, a magnesium silicate hydroxide and olivine.[4][5] Molecular sieves also function in this capacity.
Various scrubbing processes have been proposed to remove CO2 from the air, or from flue gases. These usually involve using a variant of the Kraft process. Scrubbing processes may be based on sodium hydroxide.[6][7] The CO2 is absorbed into solution, transferred to lime via a process called causticization and released in a kiln. With some modifications to the existing processes, mainly an oxygen-fired kiln, the end result is a concentrated stream of CO2 ready for storage or use in fuels. An alternative to this thermo-chemical process is an electrical one in which a nominal voltage is applied across the carbonate solution to release the CO2. While simpler, this electrical process consumes more energy as it splits water at the same time. Since it depends on electricity, the electricity needs to be renewable, like PV. Otherwise the CO2 produced during electricity production has to be taken into account. Early incarnations of air capture used electricity as the energy source; hence, were dependent on a carbon-free source. Thermal air capture systems use heat generated on-site, which reduces the inefficiencies associated with off-site electricity production, but of course it still needs a source of (carbon-free) heat. Concentrated solar power is an example of such a source.[8]
Sodium hydroxide
Zeman and Lackner outlined a specific method of air capture.[9]
First, CO2 is absorbed by an alkaline NaOH solution to produce dissolved sodium carbonate. The absorption reaction is a gas liquid reaction, strongly exothermic, (below)
- 2NaOH(aq) + CO2(g) → Na2CO3(aq) + H2O(l)
- Na2CO3(aq) + Ca(OH)2(s) →-> 2NaOH(aq) + CaCO3(s)
- ΔH° = -5.3 kJ/mol
Causticization is performed ubiquitously in the pulp and paper industry and readily transfers 94% of the carbonate ions from the sodium to the calcium cation.[9] Subsequently, the calcium carbonate precipitate is filtered from solution and thermally decomposed to produce gaseous CO2. The calcination reaction is the only endothermic reaction in the process and is shown (below).
- CaCO3(s) → CaO(s) + CO2(g)
- ΔH° = + 179.2 kJ/mol
The thermal decomposition of calcite is performed in a lime kiln fired with oxygen in order to avoid an additional gas separation step. Hydration of the lime (CaO) completes the cycle. Lime hydration is an exothermic reaction that can be performed with water or steam. Using water, it is a liquid/solid reaction as shown (below).
- CaO(s) + H2O(l) → Ca(OH)2(s)
- ΔH° = -64.5 kJ/mol
Lithium hydroxide
Other strong bases such as soda lime, sodium hydroxide, potassium hydroxide, and lithium hydroxide are able to remove carbon dioxide by chemically reacting with it. In particular, lithium hydroxide is used aboard spacecraft to remove carbon dioxide from the atmosphere. It reacts with carbon dioxide to make lithium carbonate:[10]
- 2 LiOH(s) + 2 H2O(g) → 2 LiOH.H2O(s)
- 2 LiOH.H2O(s) + CO2(g) → Li2CO3(s) + 3 H2O(g)
The net reaction being:
- 2 LiOH(s) + CO2(g) → Li2CO3(s) + H2O(g)
Lithium peroxide can also be used as it absorbs more CO2 per unit weight with the added advantage of releasing oxygen.[11] This is useful for breathing air systems and was used to remove CO2 from the Apollo spacecraft.
Regenerative carbon dioxide removal system
The regenerative carbon dioxide removal system (RCRS) on the space shuttle orbiter used a two-bed system that provided continuous removal of carbon dioxide without expendable products. Regenerable systems allowed a shuttle mission a longer stay in space without having to replenish its sorbent canisters. Older lithium hydroxide (LiOH)-based systems, which are non-regenerable, were replaced by regenerable metal-oxide-based systems. A system based on metal oxide primarily consisted of a metal oxide sorbent canister and a regenerator assembly. It worked by removing carbon dioxide using a sorbent material and then regenerating the sorbent material. The metal-oxide sorbent canister was regenerated by pumping air at approximately 400 °F (204 °C) through it at a standard flow rate of 7.5 cu ft/min (0.0035 m3/s) for 10 hours.[12]
Activated carbon
Activated carbon can be used as a carbon dioxide scrubber. Air with high carbon dioxide content, such as air from fruit storage locations, can be blown through beds of activated carbon and the carbon dioxide will adsorb onto the activated carbon. Once the bed is saturated it must then be "regenerated" by blowing low carbon dioxide air, such as ambient air, through the bed. This will release the carbon dioxide from the bed, and it can then be used to scrub again, leaving the net amount of carbon dioxide in the air the same as when the process was started.
Other methods
Many other methods and materials have been discussed for scrubbing carbon dioxide.
- Adsorption[13]
- Regenerative carbon dioxide removal system (RCRS)
- Photosynthesis: e.g. Algae based carbon sink
- Polymer membrane gas separators[14][15]
- Reversing heat exchangers
See also
- Breath
- Carbon capture and storage
- Carbon dioxide air capture
- Greenhouse gas
- Rebreather
References
- ↑ Gary T. Rochelle "Amine Scrubbing for CO2 Capture" Science volumen 325, 1652 (2009). doi:10.1126/science.1176731
- ↑ Sunho Choi, Jeffrey H. Drese, and Christopher W. Jones "Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources" ChemSusChem 2009, 2, 796 – 854. doi:10.1002/cssc.200900036
- ↑ "Imagine No Restrictions On Fossil-Fuel Usage And No Global Warming". ScienceDaily. April 15, 2002.
- ↑ "Natural Mineral Locks Up Carbon Dioxide". Sciencedaily.com. 2004-09-03. Retrieved 2011-06-01.
- ↑
- ↑ Chang, Kenneth (2008-02-19). "Scientists would turn greenhouse gas into gasoline". New York Times. Retrieved 2009-10-29.
- ↑ "Chemical 'sponge' could filter CO2 from the air - environment - 03 October 2007". New Scientist. Retrieved 2009-10-29.
- ↑ "Can technology clear the air? - environment - 12 January 2009". New Scientist. 2009-01-12. Retrieved 2009-10-29.
- ↑ 9.0 9.1 Zeman, F. S.; Lackner, K. S. (2004). "Capturing carbon dioxide directly from the atmosphere". World Resour. Rev. 16: 157–72.
- ↑ Jaunsen, JR (1989). "The Behavior and Capabilities of Lithium Hydroxide Carbon Dioxide Scrubbers in a Deep Sea Environment". US Naval Academy Technical Report. USNA-TSPR-157. Retrieved 2008-06-17.
- ↑ Petzow, G. N.; Aldinger, F.; Jönsson, S.; Welge, P.; Van Kampen, V.; Mensing, T.; Brüning, T. (2005). "Beryllium and Beryllium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a04_011.pub2. ISBN 3527306730.
- ↑ "Carbon Dioxide Removal". Hamilton Sundstrand. Archived from the original on 2007-10-31. Retrieved 2008-10-27. "The new metal-oxide-based system replaces the existing non-regenerable lithium hydroxide (LiOH) carbon dioxide (CO2) removal system located in the EMU’s Primary Life Support System."
- ↑ "Adsorption and Desorption of CO2 on Solid Sorbents" (PDF). Retrieved 2011-06-01.
- ↑
- ↑
| |||||||||||||||||||||||
%2C_is_fitted_with_a_Kirby_Morgan_37_Dive_Helmet.jpg)