Capsular contracture
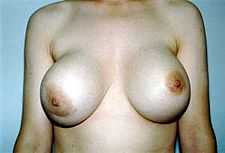
Capsular contracture is a response of the immune system to foreign materials in the human body. Medically, it occurs mostly in context of the complications from breast implants and artificial joint prosthetics.
The occurrence of capsular contraction follows the formation of capsules of tightly-woven collagen fibers, created by the immune response to the presence of foreign objects surgically installed to the human body, e.g. breast implants, artificial pacemakers, orthopedic prostheses; biological protection by isolation and toleration. Capsular contracture occurs when the collagen-fiber capsule tightens and squeezes the breast implant; as such, it is a medical complication that can be very painful and discomforting, and might distort the aesthetics of the breast implant and the breast. Although the cause of capsular contracture is unknown, factors common to its incidence include bacterial contamination, rupture of the breast-implant shell, leakage of the silicone-gel filling, and hematoma.
Moreover, because capsular contracture is a consequence of the immune system defending the patient’s bodily integrity and health, it might reoccur, even after the requisite corrective surgery for the initial incidence. The degree of an incidence of capsular contracture is graded using the four-grade Baker scale:
- Grade I — the breast is normally soft and appears natural in size and shape
- Grade II — the breast is a little firm, but appears normal
- Grade III — the breast is firm and appears abnormal
- Grade IV — the breast is hard, painful to the touch, and appears abnormal
The surgical implantation methods that have reduced capsular contracture include submuscular breast implant placement, using either textured or polyurethane-coated implants,[1][2][3] limited handling of the implants, minimal contact with the chest wall skin before their insertions, and irrigating the surgical sites with triple-antibiotic solutions.[4][5] The correction of capsular contracture might require the surgical removal (release) of the capsule, or the removal, and possible replacement, of the breast implant, itself. Closed capsulotomy (disrupting the capsule via external manipulation), a once-common maneuver for treating hard capsules, was discontinued because it might rupture the breast implant. Non-surgical methods of treating capsules include massage, external ultrasound,[6] treatment with leukotriene pathway inhibitors (e.g. Accolate, Singulair),[7][8] and pulsed electromagnetic field therapy.[9]
The Mentor Worldwide LLC corporation, one of the two, U.S. FDA-approved breast-implant device manufacturers, conducted a study of the medical complications suffered by breast implantation surgery patients. In March 2000, at a Food and Drug Administration presentation, the Mentor report indicated that 43 per cent of patients with saline breast implants reported medical complications occurring within three years of the surgery; moreover, 10 per cent of that percentage group complained of capsular contracture.[citation needed]
Sources
- Section on Complications from the FDA Breast Implant Consumer Handbook - 2004. Most of the above text was copied verbatim from this public domain source.
- Safety of Silicone Breast Implants. Institute of Medicine National Academy Press, Washington, D.C. 2000.
- Breast Augmentation and Breast Implants Information Website - ImplantInfo by Nicole
References
- ↑ Barnsley, G Philip; Sigurdson, Leif J.; Barnsley, Shannon E. (2006). "Textured Surface Breast Implants in the Prevention of Capsular Contracture among Breast Augmentation Patients: A Meta-Analysis of Randomized Controlled Trials". Plastic and Reconstructive Surgery 117 (7): 2182–90. doi:10.1097/01.prs.0000218184.47372.d5. PMID 16772915.
- ↑ Wong, Chin-Ho; Samuel, Miny; Tan, Bien-Keem; Song, Colin (2006). "Capsular Contracture in Subglandular Breast Augmentation with Textured versus Smooth Breast Implants: A Systematic Review". Plastic and Reconstructive Surgery 118 (5): 1224–36. doi:10.1097/01.prs.0000237013.50283.d2. PMID 17016195.
- ↑ Handel, N; Gutierrez, J (2006). "Long-term safety and efficacy of polyurethane foam-covered breast implants". Aesthetic Surgery Journal 26 (3): 265–74. doi:10.1016/j.asj.2006.04.001. PMID 19338905.
- ↑ Mladick, Richard A. (1993). "?No-Touch? Submuscular saline breast augmentation technique". Aesthetic Plastic Surgery 17 (3): 183–92. doi:10.1007/BF00636260. PMID 8213311.
- ↑ Adams, William P.; Haydon, M Scott; Raniere, Joseph; Trott, Suzanne; Marques, Marisa; Feliciano, Michael; Robinson, Jack B.; Tang, Liping et al. (2006). "A Rabbit Model for Capsular Contracture: Development and Clinical Implications". Plastic and Reconstructive Surgery 117 (4): 1214–9; discussion 1220–1. doi:10.1097/01.prs.0000208306.79104.18. PMID 16582789.
- ↑ Planas, Jorge; Cervelli, Valerio; Planas, Gabriel (2001). "Five-Year Experience on Ultrasonic Treatment of Breast Contractures". Aesthetic Plastic Surgery 25 (2): 89–93. doi:10.1007/s002660010102. PMID 11349308.
- ↑ Schlesinger, S; Ellenbogen, R; Desvigne, MN; Svehlak, S; Heck, R (2002). "Zafirlukast (Accolate): A new treatment for capsular contracture". Aesthetic Surgery Journal 22 (4): 329–36. doi:10.1067/maj.2002.126753. PMID 19331987.
- ↑ Scuderi, Nicolò; Mazzocchi, Marco; Fioramonti, Paolo; Bistoni, Giovanni (2006). "The Effects of Zafirlukast on Capsular Contracture: Preliminary Report". Aesthetic Plastic Surgery 30 (5): 513–20. doi:10.1007/s00266-006-0038-3. PMID 16977359.
- ↑ Silver, Harold (1982). "Reduction of Capsular Contracture with Two-Stage Augmentation Mammaplasty and Pulsed Electromagnetic Energy (Diapulse Therapy)". Plastic and Reconstructive Surgery 69 (5): 802–8. doi:10.1097/00006534-198205000-00013. PMID 7071225.