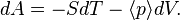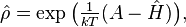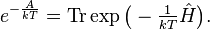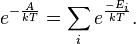Canonical ensemble
| Statistical mechanics |
|---|
 |
|
In statistical mechanics, a canonical ensemble is the statistical ensemble that is used to represent the possible states of a mechanical system which is in thermal equilibrium with a heat bath.[1] The system is said to be closed in the sense that the system can exchange energy with a heat bath, so that various possible states of the system can differ in total energy. The system's composition, volume, and shape are kept the same in all possible states of the system.
The thermodynamic variable of the canonical ensemble is the absolute temperature (symbol: T). The ensemble is also dependent on mechanical variables such as the number of particles in the system (symbol: N) and the system's volume (symbol: V), each which influence the nature of the system's internal states. This ensemble is therefore sometimes called the NVT ensemble, as each of these three quantities is a constant of the ensemble.
In simple terms, the canonical ensemble assigns a probability P to each distinct microstate given by the following exponential:
where E is the total energy of the microstate, and k is Boltzmann's constant.
The number A is the free energy (specifically, the Helmholtz free energy) and is a constant for the ensemble. However, the probabilities and A will vary if different N, V, T are selected. The free energy A serves two roles: to provide a normalization factor for the probability distribution (the probabilities, over the complete set of microstates, must add up to one); and, many important ensemble averages can be directly calculated from the function A(N, V, T).
Historically, the canonical ensemble was first described by Boltzmann (who called it a holode) in 1884 in a relatively unknown paper.[2] It was later reformulated and extensively investigated by Gibbs in 1902.[1] An alternative but equivalent formulation for the same concept writes the probability as  , using the canonical partition function
, using the canonical partition function  rather than the free energy. The equations below (in terms of free energy) may be restated in terms of the canonical partition function by simple mathematical manipulations.
rather than the free energy. The equations below (in terms of free energy) may be restated in terms of the canonical partition function by simple mathematical manipulations.
Applicability of canonical ensemble
The canonical ensemble is exactly the ensemble that describes the possible states of an isolated system that is in thermal equilibrium with a heat bath (the derivation of this fact can be found in Gibbs[1]). In this case the canonical ensemble applies exactly to systems of any size; while it is necessary to assume that the heat bath is very large (i. e., in the macroscopic limit), the system itself may be small or large.
The condition that the system is isolated is necessary in order to ensure it has well-defined equation of motion.[1] In practice, however, it is desirable to apply the canonical ensemble to describe systems that are in direct contact with the heat bath, since it is that contact that ensures the equilibrium. The use of the canonical ensemble in these cases is usually justified either 1) by assuming that the contact is weak, or 2) by incorporating a part of the heat bath connection into the system under analysis, so that the connection's influence on the region of interest is correctly modelled.
When the total energy is exactly known but the internal state of the system is otherwise completely unknown, a better description is the microcanonical ensemble. Conversely, for equilibrium systems where the particle number is not fixed (due to a connection to a particle reservoir), a better description is found in the grand canonical ensemble. For large systems (in the thermodynamic limit) these other ensembles become essentially equivalent to the canonical ensemble, at least for average quantities.
Properties
- Uniqueness: The canonical ensemble is uniquely determined for a given system at a given temperature, and does not depend on arbitrary choices such as choice of coordinate system (classical mechanics), of basis (quantum mechanics), or of the zero of energy.[1]
- Statistical equilibrium (steady state): A canonical ensemble does not evolve over time, despite the fact that the underlying system is in constant motion. This is because the ensemble is only a function of a conserved quantity of the system (energy).[1]
- Thermal equilibrium with other systems: Two systems, each described by a canonical ensemble of equal temperature, brought into thermal contact[note 1] will each retain the same ensemble and the resulting combined system is described by a canonical ensemble of the same temperature.[1]
- Maximum entropy: For a given mechanical system (fixed N, V), the canonical ensemble average −⟨log P⟩ is the maximum possible of any ensemble with the same ⟨E⟩.[1]
- Minimum free energy: For a given mechanical system (fixed N, V) and given value of T, the canonical ensemble average ⟨E + kT log P⟩ is the lowest possible of any ensemble.[1]
- The partial derivatives of the function A(N, V, T) give important canonical ensemble average quantities:
- the average pressure is[1]
- the Gibbs entropy is[1]
- the partial derivative ∂A/∂N is approximately related to chemical potential, although the concept of chemical equilibrium does not exactly apply to canonical ensembles of small systems.[note 2]
- and the average energy is[1]
- the average pressure is[1]
- Exact differential: From the above expressions, it can be seen that the function A(V, T), for a given N, has the exact differential[1]
- First law of thermodynamics: Substituting the above relationship for for ⟨E⟩ into the exact differential of A, an equation similar to the first law of thermodynamics is found, except with average signs on some of the quantities:[1]
- Energy fluctuations: The energy in the system has uncertainty in the canonical ensemble. The variance of the energy is[1]
Example ensembles
Boltzmann distribution (separable system)
If a system described by a canonical ensemble can be separated into independent parts (this happens if the different parts do not interact), and each of those parts has a fixed material composition, then each part can be seen as a system unto itself and is described by a canonical ensemble having the same temperature as the whole. Moreover, if the system is made up of multiple similar parts, then each part has exactly the same distribution as the other parts.
In this way, the canonical ensemble provides exactly the Boltzmann distribution (also known as Maxwell–Boltzmann statistics) for systems of any number of particles. In comparison, the justification of the Boltzmann distribution from the microcanonical ensemble only applies for systems with a large number of parts (that is, in the therymodynamic limit).
The Boltzmann distribution itself is one of the most important tools in applying statistical mechanics to real systems, as it massively simplifies the study of systems that can be separated into independent parts (e. g., particles in a gas, electromagnetic modes in a cavity, molecular bonds in a polymer).
Ising model (strongly interacting system)
In a system composed of pieces that interact with each other, it is usually not possible to find a way to separate the system into independent subsystems as done in the Boltzmann distribution. In these systems it is necessary to resort to using the full expression of the canonical ensemble in order to describe the thermodynamics of the system when it is thermostatted to a heat bath. The canonical ensemble is generally the most straightforward framework for studies of statistical mechanics and even allows one to obtain exact solutions in some interacting model systems.[3]
A classic example of this is the Ising model, which is a widely discussed toy model for the phenomena of ferromagnetism and of self-assembled monolayer formation, and is one of the simplest models that shows a phase transition. Lars Onsager famously calculated exactly the free energy of an infinite-sized square-lattice Ising model at zero magnetic field, in the canonical ensemble.[4]
Precise expressions for the ensemble
The precise mathematical expression for a statistical ensemble depends on the kind of mechanics under consideration—quantum or classical—since the notion of a "microstate" is considerably different in these two cases. In quantum mechanics, the canonical ensemble affords a simple description since diagonalization provides a discrete set of microstates with specific energies. The classical mechanical case is more complex as it involves instead an integral over canonical phase space, and the size of microstates in phase space can be chosen somewhat arbitrarily.
Quantum mechanical
Example of canonical ensemble for a quantum system consisting of one particle in a potential well.


A statistical ensemble in quantum mechanics is represented by a density matrix, denoted by ρ̂. In basis-free notation, the canonical ensemble is the density matrix[citation needed]
where Ĥ is the system's total energy operator (Hamiltonian), and exp() is the matrix exponential operator. The free energy A is determined by the probability normalization condition that the density matrix has a trace of one, Tr ρ̂ = 1:
The canonical ensemble can alternatively be written in a simple form using bra-ket notation, if the system's energy eigenstates and energy eigenvalues are known. Given a complete basis of energy eigenstates |ψi⟩, indexed by i, the canonical ensemble is:
where the Ei are the energy eigenvalues determined by Ĥ|ψi⟩ = Ei|ψi⟩. In other words, a set of microstates in quantum mechanics is given by a complete set of stationary states. The density matrix is diagonal in this basis, with the diagonal entries each directly giving a probability.
Classical mechanical
Example of canonical ensemble for a classical system consisting of one particle in a potential well.


In classical mechanics, a statistical ensemble is instead represented by a joint probability density function in the system's phase space, ρ(p1, … pn, q1, … qn), where the p1, … pn and q1, … qn are the canonical coordinates (generalized momenta and generalized coordinates) of the system's internal degrees of freedom. In a system of particles, the number of degrees of freedom n depends on the number of particles N in a way that depends on the physical situation. For a three dimensional gas of monoatoms (not molecules), n = 3N, however in diatomic gases there will also be rotational and vibrational degrees of freedom.
The probability density function for the canonical ensemble is:
where
- E is the energy of the system, a function of the phase (p1, … qn),
- h is an arbitrary but predetermined constant with the units of energy×time, setting the extent of one microstate and providing correct dimensions to ρ.[note 3]
- C is an overcounting correction factor, often used for particle systems where identical particles are able to change place with each other.[note 4]
- A provides a normalizing factor and is also the characteristic state function, the free energy.
Again, the value of A is determined by demanding that ρ is a normalized probability density function:
This integral is taken over the entire phase space.
In other words, a microstate in classical mechanics is a phase space region, and this region has volume hnC. This means that each microstate spans a range of energy, however this range can be made arbitrarily narrow by choosing h to be very small. The phase space integral can be converted into a summation over microstates, once phase space has been finely divided to a sufficient degree.
Notes
- ↑ Thermal contact means that the systems are made able to exchange energy through an interaction. The interaction must be weak as to not significantly disturb the systems' microstates.
- ↑ Since N is an integer, this "derivative" actually refers to a finite difference expression such as A(N) − A(N − 1), or A(N + 1) − A(N), or [A(N + 1) − A(N − 1)]/2. These finite difference expressions are equivalent only in the thermodynamic limit (very large N).
- ↑ (Historical note) Gibbs' original ensemble effectively set h = 1 [energy unit]×[time unit], leading to unit-dependence in the values of some thermodynamic quantities like entropy and chemical potential. Since the advent of quantum mechanics, h is often taken to be equal to Planck's constant in order to obtain a semiclassical correspondence with quantum mechanics.
- ↑ In a system of N identical particles, C = N! (factorial of N). This factor corrects the overcounting in phase space due to identical physical states being found in multiple locations. See the statistical ensemble article for more information on this overcounting.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 1.10 1.11 1.12 1.13 1.14 Gibbs, Josiah Willard (1902). Elementary Principles in Statistical Mechanics. New York: Charles Scribner's Sons.
- ↑ Cercignani, Carlo (1998). Ludwig Boltzmann: The Man Who Trusted Atoms. Oxford University Press. ISBN 9780198501541.
- ↑ Baxter, Rodney J. (1982). Exactly solved models in statistical mechanics. Academic Press Inc. ISBN 9780120831807.
- ↑ Onsager, L. (1944). "Crystal Statistics. I. A Two-Dimensional Model with an Order-Disorder Transition". Physical Review 65 (3–4): 117. doi:10.1103/PhysRev.65.117.
| |||||||||||||||||||||||||||||