Cancer stem cell

Cancer stem cells (CSCs) are cancer cells (found within tumors or hematological cancers) that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs are therefore tumorigenic (tumor-forming), perhaps in contrast to other non-tumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are proposed to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore, development of specific therapies targeted at CSCs holds hope for improvement of survival and quality of life of cancer patients, especially for sufferers of metastatic disease.
Existing cancer treatments have mostly been developed based on animal models, where therapies able to promote tumor shrinkage were deemed effective. However, animals could not provide a complete model of human disease. In particular, in mice, whose life spans do not exceed two years, tumor relapse is exceptionally difficult to study.
The efficacy of cancer treatments is, in the initial stages of testing, often measured by the ablation fraction of tumor mass (fractional kill). As CSCs would form a very small proportion of the tumor, this may not necessarily select for drugs that act specifically on the stem cells. The theory suggests that conventional chemotherapies kill differentiated or differentiating cells, which form the bulk of the tumor but are unable to generate new cells. A population of CSCs, which gave rise to it, could remain untouched and cause a relapse of the disease.
Models for tumor propagation
In different tumor subtypes, cells within the tumor population exhibit functional heterogeneity, and tumors are formed from cells with various proliferative and differentiate capacities.[1] This functional heterogeneity among cancer cells has led to the creation of at least two models, which have been put forward to account for heterogeneity and differences in tumor-regenerative capacity: the cancer stem cell (CSC) and clonal evolution models[2]


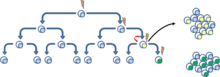

Evidence of CSC
The existence of CSCs is a subject of debate within medical research, because many studies have not been successful in discovering the similarities and differences between normal tissue stem cells and cancer (stem) cells.[7] Cancer cells must be capable of continuous proliferation and self-renewal in order to retain the many mutations required for carcinogenesis, and to sustain the growth of a tumor since differentiated cells (constrained by the Hayflick Limit[8]) cannot divide indefinitely. However, it is debated whether such cells represent a minority. If most cells of the tumor are endowed with stem cell properties, there is no incentive to focus on a specific subpopulation. There is also debate on the cell of origin of CSCs - whether they originate from normal stem cells that have lost the ability to regulate proliferation, or from more differentiated population of progenitor cells that have acquired abilities to self-renew (which is related to the issue of stem cell plasticity).
The first conclusive evidence for CSCs was published in 1997 in Nature Medicine. Bonnet and Dick[9] isolated a subpopulation of leukaemic cells that expressed a specific surface marker CD34, but lacked the CD38 marker. The authors established that the CD34+/CD38- subpopulation is capable of initiating tumors in NOD/SCID mice that are histologically similar to the donor.
In cancer research experiments, tumor cells are sometimes injected into an experimental animal to establish a tumor. Disease progression is then followed in time and novel drugs can be tested for their ability to inhibit it. However, efficient tumor formation requires thousands or tens of thousands of cells to be introduced. Classically, this has been explained by poor methodology (i.e. the tumor cells lose their viability during transfer) or the critical importance of the microenvironment, the particular biochemical surroundings of the injected cells. Supporters of the CSC paradigm argue that only a small fraction of the injected cells, the CSCs, have the potential to generate a tumor. In human acute myeloid leukemia the frequency of these cells is less than 1 in 10,000.[9]
Further evidence comes from histology, the study of the tissue structure of tumors. Many tumors are very heterogeneous and contain multiple cell types native to the host organ. Heterogeneity is commonly retained by tumor metastases. This implies that the cell that produced them had the capacity to generate multiple cell types. In other words, it possessed multidifferentiative potential, a classical hallmark of stem cells.[9]
The existence of leukaemic stem cells prompted further research into other types of cancer. CSCs have recently been identified in several solid tumors, including cancers of the:
- Brain[10]
- Breast[11]
- Colon[12]
- Ovary[13][14][15]
- Pancreas[16]
- Prostate[17][18]
- Melanoma[19][20][21][22]
- Multiple Myeloma[23][24]
Mechanistic and mathematical models
Once the pathways to cancer are hypothesized, it is possible to develop predictive mathematical biology models,[25] e.g., based on the cell compartment method. For instance, the growths of the abnormal cells from their normal counterparts can be denoted with specific mutation probabilities. Such a model has been employed to predict that repeated insult to mature cells increases the formation of abnormal progeny, and hence the risk of cancer.[26] Considerable work needs to be done, however, before the clinical efficacy of such models[27] is established.
Origin of CSC

The origin of cancer stem cells is still an area of ongoing research. Several camps have formed within the scientific community regarding the issue, and it is possible that several answers are correct, depending on the tumor type and the phenotype the tumor presents. One important distinction that will often be raised is that the cell of origin for a tumor can not be demonstrated using the cancer stem cell as a model. This is because cancer stem cells are isolated from end-stage tumors. Therefore, describing a cancer stem cell as a cell of origin is often an inaccurate claim, even though a cancer stem cell is capable of initiating new tumor formation.
With that caveat mentioned, various theories define the origin of cancer stem cells. In brief, CSC can be generated as: mutants in developing stem or progenitor cells, mutants in adult stem cells or adult progenitor cells, or mutant differentiated cells that acquire stem like attributes. These theories often do focus on a tumor's cell of origin and as such must be approached with skepticism.
Some researchers favor the theory that the cancer stem cell is generated by a mutation in stem cell niche populations during development. The logical progression claims that these developing stem populations are mutated and then expand such that the mutation is shared by many of the descendants of the mutated stem cell. These daughter stem cells are then much closer to becoming tumors, and since there are many of them there is more chance of a mutation that can cause cancer.[28]
Another theory associates adult stem cells with the formation of tumors. This is most often associated with tissues with a high rate of cell turnover (such as the skin or gut). In these tissues, it has long been expected that stem cells are responsible for tumor formation. This is a consequence of the frequent cell divisions of these stem cells (compared to most adult stem cells) in conjunction with the extremely long lifespan of adult stem cells. This combination creates the ideal set of circumstances for mutations to accumulate; accumulation of mutations is the primary factor that drives cancer initiation. In spite of the logical backing of the theory, only recently has any evidence appeared showing association represents an actual phenomenon. It is important to bear in mind that due to the heterogeneous nature of evidence it is possible that any individual cancer could come from an alternative origin.
A third possibility often raised is the potential de-differentiation of mutated cells such that these cells acquire stem cell like characteristics. This is often used as a potential alternative to any specific cell of origin, as it suggests that any cell might become a cancer stem cell.
Another related concept is the concept of tumor hierarchy. This concept claims that a tumor is a heterogeneous population of mutant cells, all of which share some mutations but vary in specific phenotype. In this model, the tumor is made up of several types of stem cells, one optimal to the specific environment and several less successful lines. These secondary lines can become more successful in some environments, allowing the tumor to adapt to its environment, including adaptation to tumor treatment. If this situation is accurate, it has severe repercussions on cancer stem cell specific treatment regime.[29] Within a tumor hierarchy model, it would be extremely difficult to pinpoint the cancer stem cell's origin.
Cancer stem cell isolation
CSC, now reported in most human tumors, are commonly identified and enriched using strategies for identifying normal stem cells that are similar across various studies.[30] The procedures include fluorescence-activated cell sorting (FACS), with antibodies directed at cell-surface markers, and functional approaches including SP analysis (side population assay) or Aldefluor assay.[31] The CSC-enriched population purified by these approaches is then implanted, at various cell doses, in immune-deficient mice to assess its tumor development capacity. This in vivo assay is called limiting dilution assay. The tumor cell subsets that can initiate tumor development at low cell numbers are further tested for self-renewal capacity in serial tumor studies.[32]
CSC can also be identified by efflux of incorporated Hoechst dyes via multidrug resistance (MDR) and ATP-binding cassette (ABC) Transporters.[31]
Another approach which has also been used for identification of cell subsets enriched with CSCs in vitro is sphere-forming assays. Many normal stem cells such as hematopoietics or stem cells from tissues are capable, under special culture conditions, to form three-dimensional spheres, which can differentiate into multiple cell types. As with normal stem cells, the CSCs isolated from brain or prostate tumors also have the ability to form anchorage-independent spheres.[33]
Heterogeneity (CSC markers)
Data over recent years have indicated the existence of CSCs in various solid tumors. For isolating CSCs from solid and hematological tumors, markers specific for normal stem cells of the same organ are commonly used. Nevertheless, a number of cell surface markers have proved useful for isolation of subsets enriched for CSC including CD133 (also known as PROM1), CD44, CD24, EpCAM (epithelial cell adhesion molecule, also known as epithelial specific antigen, ESA), THY1 and ATP-binding cassette B5 (ABCB5).[34]
CD133 (prominin 1) is a five-transmembrane domain glycoprotein expressed on CD34+ stem and progenitor cells, in endothelial precursors and fetal neural stem cells. It has been detected using its glycosylated epitope know as AC133.
EpCAM (epithelial cell adhesion molecule, ESA, TROP1) is hemophilic Ca2+-independent cell adhesion molecule expressed on the basolateral surface of most epithelial cells.
CD90 (THY1) is a glycosylphosphatidylinositol glycoprotein anchored in the plasma membrane and involved in signal transduction. It may also mediate adhesion between thymocytes and thymic stroma.
CD44 (PGP1) is an adhesion molecule that has pleiotropic roles in cell signaling, migration and homing. It has multiple isoforms, including CD44H, which exhibits high affinity for hyaluronate, and CD44V which has metastatic properties.
CD24 (HSA) is a glycosylated glycosylphosphatidylinositol-anchored adhesion molecule, which has co-stimulatory role in B and T cells.
ALDH is a ubiquitous aldehyde dehydrogenase family of enzymes, which catalyzes the oxidation of aromatic aldehydes to carboxyl acids. For instance, it has role in conversion of retinol to retinoic acid, which is essential for survival.[35]
The first solid malignancy from which CSCs were isolated and identified was breast cancer. Therefore, these CSCs are the most intensely studied. Breast CSCs have been enriched in CD44+CD24-/low,[34] SP,[36] ALDH+ subpopulations.[37][38] However, recent evidence indicates that breast CSCs are very phenotypically diverse, and there is evidence that not only CSC marker expression in breast cancer cells is heterogeneous but also there exist many subsets of breast CSC.[39] Last studies provide further support to this point. Both CD44+CD24- and CD44+CD24+ cell populations are tumor initiating cells; however, CSC are most highly enriched using the marker profile CD44+CD49fhiCD133/2hi.[40]
CSCs have been reported in many brain tumors. Stem-like tumor cells have been identified using cell surface markers including CD133,[41] SSEA-1 (stage-specific embryonic antigen-1),[42] EGFR[43] and CD44.[44] However, there is uncertainty about the use of CD133 for identification of brain tumor stem-like cells because tumorigenic cells are found in both CD133+ and CD133- cells in some gliomas, and some CD133+ brain tumor cells may not possess tumor-initiating capacity.[43]
Similarly, CSCs have also been reported in human colon cancer.[45] For their identification, cell surface markers such as CD133,[45] CD44[46] and ABCB5,[47] or functional analysis including clonal analysis [48] or Aldefluor assay were used.[49] Using CD133 as a positive marker for colon CSCs has generated conflicting results. Nevertheless, recent studies indicated that the AC133 epitope, but not the CD133 protein, is specifically expressed in colon CSCs and its expression is lost upon differentiation.[50] In addition, using CD44+ colon cancer cells and additional sub-fractionation of CD44+EpCAM+ cell population with CD166 enhance the success of tumor engraftments.[46]
Multiple CSCs have been reported in prostate,[51] lung and many other organs, including liver, pancreas, kidney or ovary.[52] In prostate cancer, the tumor-initiating cells have been identified in CD44+ [53] cell subset as CD44+α2β1+,[54] TRA-1-60+CD151+CD166+ [55] or ALDH+ [56] cell populations. Putative markers for lung CSCs have been reported, including CD133+,[57] ALDH+,[58] CD44+ [59] and oncofetal protein 5T4+.[60]
Metastatic cancer stem cells
Metastasis is the major cause of tumor lethality in patients. However, not every cell in the tumor has the ability to metastasize. This potential depends on factors that determine growth, angiogenesis, invasion and other basic processes of tumor cells. In the many epithelial tumors, the epithelial-mesenchymal transition (EMT) is considered as a crucial events in the metastatic process.[61] EMT and the reverse transition from mesenchymal to an epithelial phenotype (MET) are involved in embryonic development, which involves disruption of epithelial cell homeostasis and the acquisition of a migratory mesenchymal phenotype.[62] The EMT appears to be controlled by canonical pathways such as WNT and transforming growth factor β pathway.[63] The important feature of EMT is the loss of membrane E-cadherin in adherent junctions, where the β-catenin may play a significant role. Translocation of β-catenin from adherent junctions to the nucleus may lead to a loss of E-cadherin, and subsequently to EMT. There is evidence that nuclear β-catenin can directly transcriptionally activate EMT-associated target genes, such as the E-cadherin gene repressor SLUG (also known as SNAI2).[64]
Recent data have supported the concept, that tumor cells undergoing an EMT could be precursors for metastatic cancer cells, or even metastatic CSCs.[65] In the invasive edge of pancreatic carcinoma a subset of CD133+CXCR4+ (receptor for CXCL12 chemokine also known as a SDF1 ligand) cells has been defined. These cells exhibited significantly stronger migratory activity than their counterpart CD133+CXCR4- cells, but both cell subsets showed similar tumor development capacity.[66] Moreover, inhibition of the CXCR4 receptor led to the reduced metastatic potential without altering tumorigenic capacity.[67]
On the other hand, in the breast cancer CD44+CD24-/low cells are detectable in metastatic pleural effusions.[34] By contrast, an increased number of CD24+ cells have been identified in distant metastases in patients with breast cancer.[68] Although, there are only few data on mechanisms mediating metastasis in breast cancer, it is possible that CD44+CD24-/low cells initially metastasize and in the new site they change their phenotype and undergo limited differentiation.[69] These findings led to new dynamic two-phase expression pattern concept based on the existence of two forms of cancer stem cells - stationary cancer stem cells (SCS) and mobile cancer stem cells (MCS). SCS are embedded in tissue and persist in differentiated areas throughout all tumor progression. The term MCS describes cells that are located at the tumor-host interface. There is an evidence that these cells are derived from SCS through the acquisition of transient EMT [70] (Fig. 7)
Implications for cancer treatment
The existence of CSCs has several implications in terms of future cancer treatment and therapies. These include disease identification, selective drug targets, prevention of metastasis, and development of new intervention strategies.
Normal somatic stem cells are naturally resistant to chemotherapeutic agents. They produce various pumps (such as MDR[citation needed]) that pump out drugs and DNA repair proteins and they also have a slow rate of cell turnover (chemotherapeutic agents naturally target rapidly replicating cells)[citation needed]. CSCs that develop from normal stem cells may also produce these proteins, which could increase their resistance towards chemotherapeutic agents. The surviving CSCs then repopulate the tumor, causing a relapse.[71] By selectively targeting CSCs, it would be possible to treat patients with aggressive, non-resectable tumors, as well as preventing patients from metastasizing and relapsing.[71] The hypothesis suggests that upon CSC elimination, cancer could regress due to differentiation and/or cell death[citation needed]. What fraction of tumor cells are CSCs and therefore need to be eliminated is not clear yet.[72]
A number of studies have investigated the possibility of identifying specific markers that may distinguish CSCs from the bulk of the tumor (as well as from normal stem cells).[11] Proteomic and genomic signatures of tumors are also being investigated.[73][citation needed]. In 2009, scientists identified one compound, Salinomycin, that selectively reduces the proportion of breast CSCs in mice by more than 100-fold relative to Paclitaxel, a commonly used chemotherapeutic agent.[74]
The cell surface receptor interleukin-3 receptor-alpha (CD123) was shown to be overexpressed on CD34+CD38- leukemic stem cells (LSCs) in acute myelogenous leukemia (AML) but not on normal CD34+CD38- bone marrow cells.[75] Jin et al., then demonstrated that treating AML-engrafted NOD/SCID mice with a CD123-specific monoclonal antibody impaired LSCs homing to the bone marrow and reduced overal AML cell repopulation including the proportion of LSCs in secondary mouse recipients.[76]
Pathways
The design of new drugs for the treatment of CSCs will likely require an understanding of the cellular mechanisms that regulate cell proliferation. The first advances in this area were made with hematopoietic stem cells (HSCs) and their transformed counterparts in leukemia, the disease for which the origin of CSCs is best understood. It is now becoming increasingly clear that stem cells of many organs share the same cellular pathways as leukemia-derived HSCs.
Additionally, a normal stem cell may be transformed into a cancer stem cell through disregulation of the proliferation and differentiation pathways controlling it or by inducing oncoprotein activity.
BMI-1
The Polycomb group transcriptional repressor Bmi-1 was discovered as a common oncogene activated in lymphoma[77] and later shown to specifically regulate HSCs.[78] The role of Bmi-1 has also been illustrated in neural stem cells.[79] The pathway appears to be active in CSCs of pediatric brain tumors.[80]
Notch
The Notch pathway has been known to developmental biologists for decades. Its role in control of stem cell proliferation has now been demonstrated for several cell types including hematopoietic, neural and mammary[81] stem cells. Components of the Notch pathway have been proposed to act as oncogenes in mammary[82] and other tumors.
A particular branch of the Notch signaling pathway that involves the transcription factor Hes3 has been shown to regulate a number of cultured cells with cancer stem cell characteristics obtained from glioblastoma patients.[83]
Sonic hedgehog and Wnt
These developmental pathways are also strongly implicated as stem cell regulators.[84] Both Sonic hedgehog (SHH) and Wnt pathways are commonly hyperactivated in tumors and are required to sustain tumor growth. However, the Gli transcription factors that are regulated by SHH take their name from gliomas, where they are commonly expressed at high levels. A degree of crosstalk exists between the two pathways and their activation commonly goes hand-in-hand.[85] This is a trend rather than a rule. For instance, in colon cancer hedgehog signalling appears to antagonise Wnt.[86]
Sonic hedgehog blockers are available, such as cyclopamine. There is also a new water soluble cyclopamine that may be more effective in cancer treatment. There is also DMAPT, a water soluble derivative of parthenolide (induces oxidative stress, inhibits NF-κB signaling[87]) for AML (leukemia), and possibly myeloma and prostate cancer. A clinical trial of DMAPT is to start in England in late 2007 or 2008. Finally, the enzyme telomerase may qualify as a study subject in CSC physiology.[88] GRN163L (Imetelstat) was recently started in trials to target myeloma stem cells. If it is possible to eliminate the cancer stem cell, then a potential cure may be achieved if there are no more CSCs to repopulate a cancer.
References
- ↑ Heppner, GH; Miller, BE (1983). "Tumor heterogeneity: biological implications and therapeutic consequences". Cancer metastasis reviews 2 (1): 5–23. doi:10.1007/BF00046903. PMID 6616442.
- ↑ 2.0 2.1 Reya, T; Morrison, SJ; Clarke, MF; Weissman, IL (Nov 1, 2001). "Stem cells, cancer, and cancer stem cells". Nature 414 (6859): 105–11. doi:10.1038/35102167. PMID 11689955.
- ↑ Bonnet, D; Dick, JE (July 1997). "Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell". Nature Medicine 3 (7): 730–7. doi:10.1038/nm0797-730. PMID 9212098.
- ↑ Barabé, F; Kennedy, JA; Hope, KJ; Dick, JE (Apr 27, 2007). "Modeling the initiation and progression of human acute leukemia in mice". Science 316 (5824): 600–4. doi:10.1126/science.1139851. PMID 17463288.
- ↑ Nowell, PC (Oct 1, 1976). "The clonal evolution of tumor cell populations". Science 194 (4260): 23–8. doi:10.1126/science.959840. PMID 959840.
- ↑ Clark, EA; Golub, TR; Lander, ES; Hynes, RO (Aug 3, 2000). "Genomic analysis of metastasis reveals an essential role for RhoC". Nature 406 (6795): 532–5. doi:10.1038/35020106. PMID 10952316.
- ↑ Gupta PB, Chaffer CL, Weinberg RA (2009). "Cancer stem cells: mirage or reality?". Nat Med 15 (9): 1010–2. doi:10.1038/nm0909-1010. PMID 19734877.
- ↑ Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains" Exp Cell Res 37, 614-636 (1965) http://www.ncbi.nlm.nih.gov/pubmed/14315085.
- ↑ 9.0 9.1 9.2 Bonnet D, Dick JE (July 1997). "Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell". Nature Medicine 3 (7): 730–7. doi:10.1038/nm0797-730. PMID 9212098.
- ↑ Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (September 2003). "Identification of a cancer stem cell in human brain tumors". Cancer Research 63 (18): 5821–8. PMID 14522905.
- ↑ 11.0 11.1 Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (April 2003). "Prospective identification of tumorigenic breast cancer cells". Proceedings of the National Academy of Sciences of the United States of America 100 (7): 3983–8. doi:10.1073/pnas.0530291100. PMC 153034. PMID 12629218.
- ↑ O'Brien CA, Pollett A, Gallinger S, Dick JE (January 2007). "A human colon cancer cell capable of initiating tumour growth in immunodeficient mice". Nature 445 (7123): 106–10. doi:10.1038/nature05372. PMID 17122772.
- ↑ Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP (June 2008). "Identification and characterization of ovarian cancer-initiating cells from primary human tumors". Cancer Research 68 (11): 4311–20. doi:10.1158/0008-5472.CAN-08-0364. PMC 2553722. PMID 18519691.
- ↑ PMID: 19158483
- ↑ Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, Mor G. Cell Cycle. 2009 Jan 1;8(1):158-66.
- ↑ Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM (February 2007). "Identification of pancreatic cancer stem cells". Cancer Research 67 (3): 1030–7. doi:10.1158/0008-5472.CAN-06-2030. PMID 17283135.
- ↑ Maitland NJ, Collins AT (June 2008). "Prostate cancer stem cells: a new target for therapy". J. Clin. Oncol. 26 (17): 2862–70. doi:10.1200/JCO.2007.15.1472. PMID 18539965.
- ↑ Lang Sh, Frame F, Collins A (January 2009). "Prostate cancer stem cells". J. Pathol. 217 (2): 299–306. doi:10.1002/path.2478. PMC 2673349. PMID 19040209.
- ↑ Schatton T, Murphy GF, Frank, NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH (Jan 2008). "Identification of cells initiating human melanomas". Nature 451 (7176): 345–9. doi:10.1038/nature06489. PMC 3660705. PMID 18202660.
- ↑ Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT, Weissman IL (Jul 2010). "Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271". Nature 466 (7302): 133–7. doi:10.1038/nature09161. PMC 2898751. PMID 20596026.
- ↑ Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. (Feb 2011). "Eradication of melanomas by targeted elimination of a minor subset of tumor cells". PNAS 108 (6): 2474–9. doi:10.1073/pnas.1009069108. PMC 3038763. PMID 21282657.
- ↑ Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M, Sommer L. (Mar 2011). "Human CD271-Positive Melanoma Stem Cells Associated with Metastasis Establish Tumor Heterogeneity and Long-Term Growth". Cancer Res. 71 (8): 3098–109. doi:10.1158/0008-5472.CAN-10-3997. PMID 21393506.
- ↑ Matsui W, Huff CA, Wang Q, et al. (March 2004). "Characterization of clonogenic multiple myeloma cells". Blood 103 (6): 2332–6. doi:10.1182/blood-2003-09-3064. PMC 3311914. PMID 14630803.
- ↑ Matsui W, Wang Q, Barber JP, et al. (January 2008). "Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance". Cancer Res. 68 (1): 190–7. doi:10.1158/0008-5472.CAN-07-3096. PMC 2603142. PMID 18172311.
- ↑ Preziosi, Luigi (2003). Cancer Modelling and Simulation. Boca Raton: CRC Press. ISBN 1-58488-361-8.
- ↑ Ganguly R, Puri IK (February 2006). "Mathematical model for the cancer stem cell hypothesis". Cell proliferation 39 (1): 3–14. doi:10.1111/j.1365-2184.2006.00369.x. PMID 16426418.
- ↑ Ganguly R, Puri IK (June 2007). "Mathematical model for chemotherapeutic drug efficacy in arresting tumour growth based on the cancer stem cell hypothesis". Cell proliferation 40 (3): 338–354. doi:10.1111/j.1365-2184.2007.00434.x. PMID 17531079.
- ↑ Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, Lee EY, Zhu Y (June 2009). "Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model". Cancer Cell 15 (6): 514–26. doi:10.1016/j.ccr.2009.04.001. PMC 2721466. PMID 19477430.
- ↑ Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM (October 2006). "Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells". Cancer Research 66 (19): 9339–44. doi:10.1158/0008-5472.CAN-06-3126. PMID 16990346.
- ↑ Golebiewska, A; Brons, NH; Bjerkvig, R; Niclou, SP (Feb 4, 2011). "Critical appraisal of the side population assay in stem cell and cancer stem cell research". Cell stem cell 8 (2): 136–47. doi:10.1016/j.stem.2011.01.007. PMID 21295271.
- ↑ 31.0 31.1 Scharenberg, CW; Harkey, MA; Torok-Storb, B (Jan 15, 2002). "The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors". Blood 99 (2): 507–12. doi:10.1182/blood.V99.2.507. PMID 11781231.
- ↑ Pastrana, E; Silva-Vargas, V; Doetsch, F (May 6, 2011). "Eyes wide open: a critical review of sphere-formation as an assay for stem cells". Cell stem cell 8 (5): 486–98. doi:10.1016/j.stem.2011.04.007. PMC 3633588. PMID 21549325.
- ↑ Nicolis, SK (February 2007). "Cancer stem cells and "stemness" genes in neuro-oncology". Neurobiology of disease 25 (2): 217–29. doi:10.1016/j.nbd.2006.08.022. PMID 17141509.
- ↑ 34.0 34.1 34.2 Al-Hajj, M; Wicha, MS; Benito-Hernandez, A; Morrison, SJ; Clarke, MF (Apr 1, 2003). "Prospective identification of tumorigenic breast cancer cells". Proceedings of the National Academy of Sciences of the United States of America 100 (7): 3983–8. doi:10.1073/pnas.0530291100. PMC 153034. PMID 12629218.
- ↑ Visvader, JE; Lindeman, GJ (October 2008). "Cancer stem cells in solid tumours: accumulating evidence and unresolved questions". Nature Reviews Cancer 8 (10): 755–68. doi:10.1038/nrc2499. PMID 18784658.
- ↑ Hirschmann-Jax, C; Foster, AE; Wulf, GG; Nuchtern, JG; Jax, TW; Gobel, U; Goodell, MA; Brenner, MK (Sep 28, 2004). "A distinct "side population" of cells with high drug efflux capacity in human tumor cells". Proceedings of the National Academy of Sciences of the United States of America 101 (39): 14228–33. doi:10.1073/pnas.0400067101. PMC 521140. PMID 15381773.
- ↑ Ginestier, C; Hur, MH; Charafe-Jauffret, E; Monville, F; Dutcher, J; Brown, M; Jacquemier, J; Viens, P; Kleer, CG; Liu, S; Schott, A; Hayes, D; Birnbaum, D; Wicha, MS; Dontu, G (November 2007). "ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome". Cell stem cell 1 (5): 555–67. doi:10.1016/j.stem.2007.08.014. PMC 2423808. PMID 18371393.
- ↑ Pece, S; Tosoni, D; Confalonieri, S; Mazzarol, G; Vecchi, M; Ronzoni, S; Bernard, L; Viale, G; Pelicci, PG; Di Fiore, PP (Jan 8, 2010). "Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content". Cell 140 (1): 62–73. doi:10.1016/j.cell.2009.12.007. PMID 20074520.
- ↑ Deng, S; Yang, X; Lassus, H; Liang, S; Kaur, S; Ye, Q; Li, C; Wang, LP; Roby, KF; Orsulic, S; Connolly, DC; Zhang, Y; Montone, K; Bützow, R; Coukos, G; Zhang, L (Apr 21, 2010). "Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers". In Cao, Yihai. PLoS ONE 5 (4): e10277. doi:10.1371/journal.pone.0010277. PMC 2858084. PMID 20422001.
- ↑ Meyer, MJ; Fleming, JM; Lin, AF; Hussnain, SA; Ginsburg, E; Vonderhaar, BK (Jun 1, 2010). "CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor-negative breast cancer". Cancer Research 70 (11): 4624–33. doi:10.1158/0008-5472.CAN-09-3619. PMID 20484027.
- ↑ Singh, SK; Hawkins, C; Clarke, ID; Squire, JA; Bayani, J; Hide, T; Henkelman, RM; Cusimano, MD; Dirks, PB (Nov 18, 2004). "Identification of human brain tumour initiating cells". Nature 432 (7015): 396–401. doi:10.1038/nature03128. PMID 15549107.
- ↑ Son, MJ; Woolard, K; Nam, DH; Lee, J; Fine, HA (May 8, 2009). "SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma". Cell stem cell 4 (5): 440–52. doi:10.1016/j.stem.2009.03.003. PMID 19427293.
- ↑ 43.0 43.1 Mazzoleni, S; Politi, LS; Pala, M; Cominelli, M; Franzin, A; Sergi Sergi, L; Falini, A; De Palma, M; Bulfone, A; Poliani, PL; Galli, R (Oct 1, 2010). "Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis". Cancer Research 70 (19): 7500–13. doi:10.1158/0008-5472.CAN-10-2353. PMID 20858720.
- ↑ Anido, J; Sáez-Borderías, A; Gonzàlez-Juncà, A; Rodón, L; Folch, G; Carmona, MA; Prieto-Sánchez, RM; Barba, I; Martínez-Sáez, E; Prudkin, L; Cuartas, I; Raventós, C; Martínez-Ricarte, F; Poca, MA; García-Dorado, D; Lahn, MM; Yingling, JM; Rodón, J; Sahuquillo, J; Baselga, J; Seoane, J (Dec 14, 2010). "TGF-β Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma". Cancer Cell 18 (6): 655–68. doi:10.1016/j.ccr.2010.10.023. PMID 21156287.
- ↑ 45.0 45.1 O'Brien, CA; Pollett, A; Gallinger, S; Dick, JE (Jan 4, 2007). "A human colon cancer cell capable of initiating tumour growth in immunodeficient mice". Nature 445 (7123): 106–10. doi:10.1038/nature05372. PMID 17122772.
- ↑ 46.0 46.1 Dalerba, P; Dylla, SJ; Park, IK; Liu, R; Wang, X; Cho, RW; Hoey, T; Gurney, A; Huang, EH; Simeone, DM; Shelton, AA; Parmiani, G; Castelli, C; Clarke, MF (Jun 12, 2007). "Phenotypic characterization of human colorectal cancer stem cells". Proceedings of the National Academy of Sciences of the United States of America 104 (24): 10158–63. doi:10.1073/pnas.0703478104. PMC 1891215. PMID 17548814.
- ↑ Wilson, BJ; Schatton, T; Zhan, Q; Gasser, M; Ma, J; Saab, KR; Schanche, R; Waaga-Gasser, AM; Gold, JS; Huang, Q; Murphy, GF; Frank, MH; Frank, NY (Aug 1, 2011). "ABCB5 identifies a therapy-refractory tumor cell population in colorectal cancer patients". Cancer Research 71 (15): 5307–16. doi:10.1158/0008-5472.CAN-11-0221. PMC 3395026. PMID 21652540.
- ↑ Odoux, C; Fohrer, H; Hoppo, T; Guzik, L; Stolz, DB; Lewis, DW; Gollin, SM; Gamblin, TC; Geller, DA; Lagasse, E (Sep 1, 2008). "A stochastic model for cancer stem cell origin in metastatic colon cancer". Cancer Research 68 (17): 6932–41. doi:10.1158/0008-5472.CAN-07-5779. PMC 2562348. PMID 18757407.
- ↑ Huang, EH; Hynes, MJ; Zhang, T; Ginestier, C; Dontu, G; Appelman, H; Fields, JZ; Wicha, MS; Boman, BM (Apr 15, 2009). "Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis". Cancer Research 69 (8): 3382–9. doi:10.1158/0008-5472.CAN-08-4418. PMC 2789401. PMID 19336570.
- ↑ Kemper, K; Sprick, MR; de Bree, M; Scopelliti, A; Vermeulen, L; Hoek, M; Zeilstra, J; Pals, ST; Mehmet, H; Stassi, G; Medema, JP (Jan 15, 2010). "The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation". Cancer Research 70 (2): 719–29. doi:10.1158/0008-5472.CAN-09-1820. PMID 20068153.
- ↑ Liu, C; Kelnar, K; Liu, B; Chen, X; Calhoun-Davis, T; Li, H; Patrawala, L; Yan, H; Jeter, C; Honorio, S; Wiggins, JF; Bader, AG; Fagin, R; Brown, D; Tang, DG (February 2011). "The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44". Nature Medicine 17 (2): 211–5. doi:10.1038/nm.2284. PMC 3076220. PMID 21240262.
- ↑ Ho, MM; Ng, AV; Lam, S; Hung, JY (May 15, 2007). "Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells". Cancer Research 67 (10): 4827–33. doi:10.1158/0008-5472.CAN-06-3557. PMID 17510412.
- ↑ Patrawala, L; Calhoun, T; Schneider-Broussard, R; Li, H; Bhatia, B; Tang, S; Reilly, JG; Chandra, D; Zhou, J; Claypool, K; Coghlan, L; Tang, DG (Mar 16, 2006). "Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells". Oncogene 25 (12): 1696–708. doi:10.1038/sj.onc.1209327. PMID 16449977.
- ↑ Dubrovska, A; Kim, S; Salamone, RJ; Walker, JR; Maira, SM; García-Echeverría, C; Schultz, PG; Reddy, VA (Jan 6, 2009). "The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations". Proceedings of the National Academy of Sciences of the United States of America 106 (1): 268–73. doi:10.1073/pnas.0810956106. PMC 2629188. PMID 19116269.
- ↑ Rajasekhar, VK; Studer, L; Gerald, W; Socci, ND; Scher, HI (Jan 18, 2011). "Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-κB signalling". Nature communications 2 (1): 162–. doi:10.1038/ncomms1159. PMC 3105310. PMID 21245843.
- ↑ Li, T; Su, Y; Mei, Y; Leng, Q; Leng, B; Liu, Z; Stass, SA; Jiang, F (February 2010). "ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients' outcome". Laboratory investigation; a journal of technical methods and pathology 90 (2): 234–44. doi:10.1038/labinvest.2009.127. PMC 3552330. PMID 20010854.
- ↑ Eramo, A; Lotti, F; Sette, G; Pilozzi, E; Biffoni, M; Di Virgilio, A; Conticello, C; Ruco, L; Peschle, C; De Maria, R (March 2008). "Identification and expansion of the tumorigenic lung cancer stem cell population". Cell death and differentiation 15 (3): 504–14. doi:10.1038/sj.cdd.4402283. PMID 18049477.
- ↑ Sullivan, JP; Spinola, M; Dodge, M; Raso, MG; Behrens, C; Gao, B; Schuster, K; Shao, C; Larsen, JE; Sullivan, LA; Honorio, S; Xie, Y; Scaglioni, PP; DiMaio, JM; Gazdar, AF; Shay, JW; Wistuba, II; Minna, JD (Dec 1, 2010). "Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling". Cancer Research 70 (23): 9937–48. doi:10.1158/0008-5472.CAN-10-0881. PMC 3058307. PMID 21118965.
- ↑ Leung, EL; Fiscus, RR; Tung, JW; Tin, VP; Cheng, LC; Sihoe, AD; Fink, LM; Ma, Y; Wong, MP (Nov 19, 2010). "Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties". In Jin, Dong-Yan. PLoS ONE 5 (11): e14062. doi:10.1371/journal.pone.0014062. PMC 2988826. PMID 21124918.
- ↑ Damelin, M; Geles, KG; Follettie, MT; Yuan, P; Baxter, M; Golas, J; DiJoseph, JF; Karnoub, M; Huang, S; Diesl, V; Behrens, C; Choe, SE; Rios, C; Gruzas, J; Sridharan, L; Dougher, M; Kunz, A; Hamann, PR; Evans, D; Armellino, D; Khandke, K; Marquette, K; Tchistiakova, L; Boghaert, ER; Abraham, RT; Wistuba, II; Zhou, BB (Jun 15, 2011). "Delineation of a cellular hierarchy in lung cancer reveals an oncofetal antigen expressed on tumor-initiating cells". Cancer Research 71 (12): 4236–46. doi:10.1158/0008-5472.CAN-10-3919. PMID 21540235.
- ↑ Thiery, JP (June 2002). "Epithelial-mesenchymal transitions in tumour progression". Nature Reviews Cancer 2 (6): 442–54. doi:10.1038/nrc822. PMID 12189386.
- ↑ Angerer, LM; Angerer, RC (June 1999). "Regulative development of the sea urchin embryo: signalling cascades and morphogen gradients". Seminars in cell & developmental biology 10 (3): 327–34. doi:10.1006/scdb.1999.0292. PMID 10441547.
- ↑ Mani, SA; Yang, J; Brooks, M; Schwaninger, G; Zhou, A; Miura, N; Kutok, JL; Hartwell, K; Richardson, AL; Weinberg, RA (Jun 12, 2007). "Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers". Proceedings of the National Academy of Sciences of the United States of America 104 (24): 10069–74. doi:10.1073/pnas.0703900104. PMC 1891217. PMID 17537911.
- ↑ Conacci-Sorrell, M; Simcha, I; Ben-Yedidia, T; Blechman, J; Savagner, P; Ben-Ze'ev, A (Nov 24, 2003). "Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK". The Journal of Cell Biology 163 (4): 847–57. doi:10.1083/jcb.200308162. PMC 2173691. PMID 14623871.
- ↑ Kaplan, RN; Riba, RD; Zacharoulis, S; Bramley, AH; Vincent, L; Costa, C; MacDonald, DD; Jin, DK; Shido, K; Kerns, SA; Zhu, Z; Hicklin, D; Wu, Y; Port, JL; Altorki, N; Port, ER; Ruggero, D; Shmelkov, SV; Jensen, KK; Rafii, S; Lyden, D (Dec 8, 2005). "VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche". Nature 438 (7069): 820–7. doi:10.1038/nature04186. PMC 2945882. PMID 16341007.
- ↑ Hermann, PC; Huber, SL; Herrler, T; Aicher, A; Ellwart, JW; Guba, M; Bruns, CJ; Heeschen, C (Sep 13, 2007). "Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer". Cell stem cell 1 (3): 313–23. doi:10.1016/j.stem.2007.06.002. PMID 18371365.
- ↑ Yang, ZF; Ho, DW; Ng, MN; Lau, CK; Yu, WC; Ngai, P; Chu, PW; Lam, CT; Poon, RT; Fan, ST (February 2008). "Significance of CD90+ cancer stem cells in human liver cancer". Cancer Cell 13 (2): 153–66. doi:10.1016/j.ccr.2008.01.013. PMID 18242515.
- ↑ Shipitsin, M; Campbell, LL; Argani, P; Weremowicz, S; Bloushtain-Qimron, N; Yao, J; Nikolskaya, T; Serebryiskaya, T; Beroukhim, R; Hu, M; Halushka, MK; Sukumar, S; Parker, LM; Anderson, KS; Harris, LN; Garber, JE; Richardson, AL; Schnitt, SJ; Nikolsky, Y; Gelman, RS; Polyak, K (March 2007). "Molecular definition of breast tumor heterogeneity". Cancer Cell 11 (3): 259–73. doi:10.1016/j.ccr.2007.01.013. PMID 17349583.
- ↑ Shmelkov, SV; Butler, JM; Hooper, AT; Hormigo, A; Kushner, J; Milde, T; St Clair, R; Baljevic, M; White, I; Jin, DK; Chadburn, A; Murphy, AJ; Valenzuela, DM; Gale, NW; Thurston, G; Yancopoulos, GD; D'Angelica, M; Kemeny, N; Lyden, D; Rafii, S (June 2008). "CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors". The Journal of Clinical Investigation 118 (6): 2111–20. doi:10.1172/JCI34401. PMC 2391278. PMID 18497886.
- ↑ Brabletz, T; Jung, A; Spaderna, S; Hlubek, F; Kirchner, T (September 2005). "Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression". Nature Reviews Cancer 5 (9): 744–9. doi:10.1038/nrc1694. PMID 16148886.
- ↑ 71.0 71.1 Mraz, M.; Zent, C. S.; Church, A. K.; Jelinek, D. F.; Wu, X.; Pospisilova, S.; Ansell, S. M.; Novak, A. J.; Kay, N. E.; Witzig, T. E.; Nowakowski, G. S. (2011). "Bone marrow stromal cells protect lymphoma B-cells from rituximab-induced apoptosis and targeting integrin α-4-β-1 (VLA-4) with natalizumab can overcome this resistance". British Journal of Haematology 155 (1): 53–64. doi:10.1111/j.1365-2141.2011.08794.x. PMID 21749361.
- ↑ Dirks, P (Jul 1, 2010). "Cancer stem cells: Invitation to a second round". Nature 466 (7302): 40–1. doi:10.1038/466040a. PMID 20596007.
- ↑ "Insights on neoplastic stem cells from gel-based proteomics of childhood germ cell tumors".
- ↑ Gupta, PB; Onder, TT; Jiang, G; Tao, K; Kuperwasser, C; Weinberg, RA; Lander, ES (Aug 21, 2009). "Identification of selective inhibitors of cancer stem cells by high-throughput screening". Cell 138 (4): 645–59. doi:10.1016/j.cell.2009.06.034. PMID 19682730.
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/11021753
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/19570512
- ↑ Haupt Y, Bath ML, Harris AW, Adams JM (November 1993). "bmi-1 transgene induces lymphomas and collaborates with myc in tumorigenesis". Oncogene 8 (11): 3161–4. PMID 8414519.
- ↑ Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF (May 2003). "Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells". Nature 423 (6937): 302–5. doi:10.1038/nature01587. PMID 12714971.
- ↑ Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ (October 2003). "Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation". Nature 425 (6961): 962–7. doi:10.1038/nature02060. PMC 2614897. PMID 14574365.
- ↑ Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI (December 2003). "Cancerous stem cells can arise from pediatric brain tumors". Proceedings of the National Academy of Sciences of the United States of America 100 (25): 15178–83. doi:10.1073/pnas.2036535100. PMC 299944. PMID 14645703.
- ↑ Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS (2004). "Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells". Breast cancer research : BCR 6 (6): R605–15. doi:10.1186/bcr920. PMC 1064073. PMID 15535842.
- ↑ Diévart A, Beaulieu N, Jolicoeur P (October 1999). "Involvement of Notch1 in the development of mouse mammary tumors". Oncogene 18 (44): 5973–81. doi:10.1038/sj.onc.1202991. PMID 10557086.
- ↑ Park DM, Jung J, Masjkur J, et al. (2013). "Hes3 regulates cell number in cultures from glioblastoma multiforme with stem cell characteristics". Sci Rep 3: 1095. doi:10.1038/srep01095. PMC 3566603. PMID 23393614.
- ↑ Beachy PA, Karhadkar SS, Berman DM (November 2004). "Tissue repair and stem cell renewal in carcinogenesis". Nature 432 (7015): 324–31. doi:10.1038/nature03100. PMID 15549094.
- ↑ Zhou BP, Hung MC (June 2005). "Wnt, hedgehog and snail: sister pathways that control by GSK-3beta and beta-Trcp in the regulation of metastasis". Cell cycle (Georgetown, Tex.) 4 (6): 772–6. doi:10.4161/cc.4.6.1744. PMID 15917668.
- ↑ Akiyoshi T, Nakamura M, Koga K, Nakashima H, Yao T, Tsuneyoshi M, Tanaka M, Katano M (July 2006). "Gli1, downregulated in colorectal cancers, inhibits proliferation of colon cancer cells involving Wnt signalling activation". Gut 55 (7): 991–9. doi:10.1136/gut.2005.080333. PMC 1856354. PMID 16299030.
- ↑ She M, Chen X (2009). "Targeting signal pathways active in cancer stem cells to overcome drug resistance". Chin J Lung Cancer 12 (1): 3–7. doi:10.3779/j.issn.1009-3419.2009.01.001. PMID 20712949.
- ↑ Bollmann FM (August 2008). "The many faces of telomerase: emerging extratelomeric effects". BioEssays 30 (8): 728–32. doi:10.1002/bies.20793. PMID 18623070.
Further reading
- Polyak K, Weinberg RA (April 2009). "Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits". Nature Reviews Cancer 9 (4): 265–73. doi:10.1038/nrc2620. PMID 19262571.
- Sánchez-García I, Vicente-Dueñas C, Cobaleda C (December 2007). "The theoretical basis of cancer-stem-cell-based therapeutics of cancer: can it be put into practice?". BioEssays 29 (12): 1269–80. doi:10.1002/bies.20679. PMID 18022789.
- Gao JX (2008). "Cancer stem cells: the lessons from pre-cancerous stem cells". Journal of Cellular and Molecular Medicine 12 (1): 67–96. doi:10.1111/j.1582-4934.2007.00170.x. PMID 18053092.
External links
| Wikimedia Commons has media related to Cancer stem cells. |
- European Cancer Stem Cell Research Institute A new Institute dedicated to research into cancer stem cells and related work.
- Cancer Stem Cell News A blog of news items related to cancer stem cells, with an emphasis on recent research and articles that are openly accessible
- Exploring the role of cancer stem cells in radioresistance Abstract of a review by Michael Baumann, Mechthild Krause, Richard Hill, "Nature Reviews Cancer " 2008(Jul); 8(7) 545-54
- "A Tumor's Lifeblood", Jessica Gorman, CR magazine, Summer 2006
- "Cancer Stem Cell Scientific Literature Review", UMDNJ Stem Cell Research and Regenerative Medicine, June 17, 2006
- "Stem cells may cause some forms of bone cancer", News-Medical.Net, December 7, 2005
- "The Bad Seed: Rare stem cells appear to drive cancers", Science News Online, March 20, 2004
- "The Real Problem in Breast Tumors: Cancer Stem Cells", Genome News Network, March 7, 2003
- Differentiation Therapy - A Different Approach to Treating Tumors (from Beaker Blog)
- Characteristics of Cancer Cells Cancer Inform Blog
- Cancer stem cells may be cause of brain tumors (research of John A. Boockvar)
| |||||||||||