C4mim
| 1-n-Butyl-3-methylimidazolium | ||
|---|---|---|
 | ||
| Identifiers | ||
| PubChem | 2734162 | |
| ChemSpider | 2015918 | |
| ChEBI | CHEBI:61333 | |
| ChEMBL | CHEMBL1198608 | |
| Jmol-3D images | {{#if:CCCC[n+]1ccn(C)c1|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C 8H 15N 2+ | |
| Molar mass | 139.2181 g mol-1 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
C4mim is a shorthand for 1-n-butyl-3-methylimidazolium salts. These are ionic liquids based on imidazole chemistry. A common example of such is [C4mim][Cl], or 1-n-butyl-3-methylimidazolium chloride. These salts are currently of interest in industry due their ability to be infinitely recycled and their amenability to solvation at room temperature, making them excellent green solvents. C4mim is based on the parent compound 1-butyl-3-methylimidazole with one electron removed from the imidazole arene group. The stability of this cation lies in the fact that the resulting electronic vacancy is delocalised across the arene group, albeit unequally so.
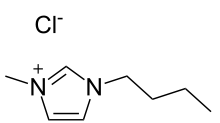 Chemical structure of 1-n-butyl-3-methylimidazolium chloride
Chemical structure of 1-n-butyl-3-methylimidazolium chloride