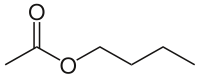
Butyl acetate
| n-Butyl acetate | |
|---|---|
 | |
 | |
| IUPAC name Butyl acetate | |
| Systematic name Butyl ethanoate | |
| Other names · Acetic acid, n-butyl ester | |
| Identifiers | |
| CAS number | 123-86-4 |
| PubChem | 31272 |
| ChemSpider | 29012 |
| UNII | 464P5N1905 |
| KEGG | C12304 |
| ChEBI | CHEBI:31328 |
| ChEMBL | CHEMBL284391 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C6H12O2 |
| Molar mass | 116.16 g mol−1 |
| Appearance | Colorless liquid with fruity odor |
| Density | 0.88 g/cm3[1] |
| Melting point | −77 °C; −107 °F; 196 K ([1]) |
| Boiling point | 127 °C; 261 °F; 400 K ([1]) |
| Solubility in water | 10 g/L (20.0 °C)[1] |
| Refractive index (nD) | 1.394 (20.0 °C) |
| Hazards | |
| Main hazards | Flammable |
| Flash point | 27 °C (81 °F)[1] |
| Related compounds | |
| Related acetates | propyl acetate amyl acetate |
| Related compounds | butanol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
n-Butyl acetate, also known as butyl ethanoate, is an organic compound commonly used as a solvent in the production of lacquers and other products. It is a colorless flammable liquid. Butyl acetate is found in many types of fruit, where along with other chemicals it imparts characteristic flavors and has a sweet smell of banana or apple. It is used as a synthetic fruit flavoring in foods such as candy, ice cream, cheeses, and baked goods.
The other three isomers of butyl acetate are: isobutyl acetate, tert-butyl acetate, and sec-butyl acetate.
Production
Butyl acetates are commonly manufactured by the Fischer esterification of a butanol isomer and acetic acid with the presence of catalytic sulfuric acid under reflux conditions.[2]
Occurrence in nature
Apples, especially of the Red Delicious variety, are flavored in part by this chemical. The alarm pheromones emitted by the Koschevnikov gland of honey bees contain butyl acetate.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Record in the GESTIS Substance Database from the IFA
- ↑ Acetic acid. (2003). In Ullman's encyclopedia of industrial chemistry (6th ed., Vol. 1, pp. 170-171). Weinheim, Germany: Wiley-VCH.
External links
- Ethylene and other chemicals in fruit
- Material Safety Data Sheet
- CDC - NIOSH Pocket Guide to Chemical Hazards