Bromley equation
The Bromley equation[1] was developed in 1973 with the objective of calculating activity coefficients for aqueous electrolyte solutions whose concentrations are above the range of validity of the Debye–Hückel equation This equation, together with Specific ion interaction theory (SIT) and Pitzer equations[2] is important for the understanding of the behaviour of ions dissolved in natural waters such as rivers, lakes and sea-water.[3][4][5]
Description
Guggenheim had proposed an extension of the Debye-Hückel equation which is the basis of SIT theory.[6] The equation can be written, in its simplest form for a 1:1 electrolyte, MX, as
 is the mean molal activity coefficient. The first term on the right-hand side is the Debye–Hückel term, with a constant, A, and the ionic strength I. β is an interaction coefficient and b the molality of the electrolyte. As the concentration decreases so the second term becomes less important until, at very low concentrations,the Debye-Hückel equation gives a satisfactory account of the activity coefficient.
is the mean molal activity coefficient. The first term on the right-hand side is the Debye–Hückel term, with a constant, A, and the ionic strength I. β is an interaction coefficient and b the molality of the electrolyte. As the concentration decreases so the second term becomes less important until, at very low concentrations,the Debye-Hückel equation gives a satisfactory account of the activity coefficient.
Bromley observed that experimental values of 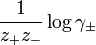 were often approximately proportional to ionic strength. Accordingly he developed the equation, for a salt of general formula
were often approximately proportional to ionic strength. Accordingly he developed the equation, for a salt of general formula 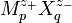
At 25 °C Aγ is equal to 0.511 and ρ is equal to one. Bromley tabulated values if the interaction coefficient B. He noted that the equation gave satisfactory agreement with experimental data up to ionic strength of 6 molal, though with decreasing precision when extrapolating to very high ionic strength. As with other equations, it is not satisfactory when there is ion-association as, for example, with divalent metal sulfates. Bromley also found that B could be expressed in terms of single-ion quantities as
where the + subscript refers to a cation and the minus subscript refers to an anion. Bromley's equation can easily be transformed for the calculation of osmotic coefficients, and Bromley also proposed extensions to multicomponent solutions and for the effect of temperature change.[1]
A modified version of the Bromley equation has been used extensively by Madariaga and co-workers.[7] In a comparison of Bromley, SIT and Pitzer models, little difference was found in the quality of fit.[8] The Bromley equation is essentially an empirical equation. The B parameters are relatively easy to determine. However, SIT theory, as extended by Scatchard.[9][10] and Ciavatta[11] is much more widely used.
By contrast the Pitzer equation is based on rigorous thermodynamics.[2] The determination Pitzer parameters is more laborious. Whilst the Bromley and SIT approaches are based on pair-wise interactions between oppositely charged ions, the Pitzer approach also allows for interactions between three ions. These equations are important for the understanding of the behaviour of ions in natural waters such as rivers, lakes and sea-water.
For some complex electrolytes, Ge et al.[12] obtained the new set of Bromley parameters using up-to-date measured or critically reviewed osmotic coefficient or activity coefficient data.
See also
- Davies equation
- Van't Hoff factor
References
- ↑ 1.0 1.1 Bromley, L.A. (1973). "Thermodynamic properties of strong electrolytes in aqueous solutions". A.I.Ch.E. 19 (2): 313–320. doi:10.1002/aic.690190216.
- ↑ 2.0 2.1 Pitzer, K.S.(editor) (1991). Activity coefficients in electrolyte solutions (2nd ed.). C.R,C. Press. ISBN 0-8493-5415-3.Chapter 3. Pitzer, K.S. Ion interaction approach: theory and data correlation, pp75-153.
- ↑ Stumm, W.; Morgan, J.J. (1996). Water Chemistry. New York: Wiley. ISBN 0-471-05196-9.
- ↑ Snoeyink, V.L.; Jenkins, D. (1980). Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. New York: Wiley. ISBN 0-471-51185-4.
- ↑ Millero, F.J. (2006). Chemical Oceanography (3rd ed.). London: Taylor and Francis. ISBN 0-8493-2280-4.
- ↑ Guggenheim, E.A.; Turgeon, J.C. (1955). "Specific interaction of ions". Trans. Faraday Soc. 51: 747–761. doi:10.1039/TF9555100747.
- ↑ Raposo, J.C.; Zuloaga, O.; Olazabel, M.-A.; Madariaga, J.M. (2003). "Development of a modified Bromley methodology for the estimation of ionic media effects on solution equilibria: Part 6. The chemical model of phosphoric acid in aqueous solution at 25 °C and comparison with arsenic acid". Fluid Phase Equilibria 207 (1-2): 69–80. doi:10.1016/S0378-3812(02)00332-1.
- ↑ Foti, C.; Gianguzza, A.; Sammartano,S. (1997). "A comparison of equations for fitting protonation constants of carboxylic acids in aqueous tetramethylammonium chloride at various ionic strengths". J. Solution Chem. 26 (6): 631. doi:10.1007/BF02767633.
- ↑ Scatchard, G. (1933). "The Coming Age of the Interionic Attraction Theory". Chem. Rev., 13 (1): 7–27. doi:10.1021/cr60044a002.
- ↑ Scatchard, G. (1936). "Concentrated solutions of strong electrolytes". Chem. Rev. 19: 309–327. doi:10.1021/cr60064a008.
- ↑ Ciavatta, L. (1980). "The specific interaction theory in the evaluating ionic equilibria". Ann. Chim. (Rome) 70: 551–562.
- ↑ X. Ge, M. Zhang, M. Guo, X. Wang. Correlation and Prediction of thermodynamic properties of Some Complex Aqueous Electrolytes by the Modified Three-Characteristic-Parameter Correlation Model. J. Chem. Eng. Data. 53(2008)950-958.http://pubs.acs.org/doi/abs/10.1021/je7006499


