Bridging ligand

A bridging ligand is a ligand that connects two or more atoms, usually metal ions.[2] The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals.
In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek character 'mu', μ,[3] with a superscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging, are called terminal ligands (see figure).
List of bridging inorganic ligands
Virtually all ligands are known to bridge, with the exception of amines and ammonia.[4] Common inorganic bridging ligands include most of the common anions.
| bridging ligand | name | example |
|---|---|---|
| OH− | hydroxide | [Fe2(OH)2(H2O)8]4+, see olation |
| O2− | oxide | [Cr2O7]2-, see polyoxometalate |
| SH− | hydrosulfido | Cp2Mo2(SH)2S2 |
| NH2− | amido | HgNH2Cl |
| N3− | nitride | [Ir3N(SO4)6(H2O)3]4-, see metal nitrido complex |
| CO | carbonyl | Fe2(CO)9, see metal carbonyl#Bridging carbonyls |
| Cl- | Chloride | Nb2Cl10, see metal halide#Halide ligands |
| H- | Hydride | B2H6 |
| CN- | Cyanide | approx. Fe7(CN)18, see cyanometalate |
Many simple organic ligands form strong bridges between metal centers. Many common examples include organic derivatives of the above inorganic ligands (R = alkyl, aryl): OR−, SR−, NR2−, NR2− (imido), PR2− (phosphido, note the ambiguity with the preceding entry), PR2− (phosphinidino), and many more.
Examples
- Compounds and Complexes with Bridging Ligands
-

In this ruthenium complex ((benzene)ruthenium dichloride dimer), two chloride ligands are terminal and two are μ2 bridging.
-

Pyrazine is a bridging ligand in this diruthenium compounds, called the Creutz-Taube complex.
-

In the cobalt cluster Co3(CO)9)Ct-Bu), the Ct-Bu ligand is triply bridging, although this aspect is typically not indicated in the formula.
-
12lessFe-Fe.png)
In triiron dodecarbonyl, two CO ligands are bridging and ten are terminal ligands. The terminal and bridging CO ligands interchange rapidly.
-
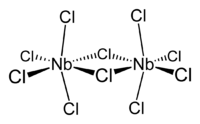
In NbCl5, there are two bridging and eight terminal chloride ligands.
-
6.png)
The cluster [Au6C(PPh3)6]2+ features μ6-carbide ligand, although again, the designator "μ" is not usually used.
-

In rhenium trioxide, the oxide ligands are all μ2. These oxide ligands "glue" together the metal centres.
-

In the case of ZrCl4, there are both terminal and doubly bridging chloride ligands.
Bonding
For doubly bridging (μ2-) ligands, two limiting representation are 4e and 2e bonding interactions. These cases are illustrated in main group chemistry by [Me2Alμ2-Cl]2 and [Me2Al(μ2-Me]2. Complicating this analysis is the possibility of metal-metal bonding. Computational studies suggest that metal-metal bonding is absent in many compounds where the metals are separated by bridging ligands. For example, calculations suggest that Fe2(CO)9 lacks a Fe-Fe bond by virtue of a 3-center, 2-electron bond involving one of three bridging CO ligands.[1]

{clear left}
Polyfunctional ligands
Polyfunctional ligands can attach to metals in many ways and thus can bridge metals in diverse ways, including sharing of one atom or using several atoms. Examples of such polyatomic ligands are the oxoanions CO32− and the related Carboxylate, PO43−, and the polyoxometallates. Several organophosphorus ligands have been developed that bridge pairs of metals, a well-known example being Ph2PCH2PPh2.
See also
- Bridging carbonyl
References
- ↑ 1.0 1.1 Jennifer C. Green, Malcolm L. H. Green, Gerard Parkin "The occurrence and representation of three-centre two-electron bonds in covalent inorganic compounds" Chem. Commun. 2012, 11481-11503. doi:10.1039/c2cc35304k
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "bridging ligand".
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "µ- (mu)".
- ↑ Werner, H. (2004). "The Way into the Bridge: A New Bonding Mode of Tertiary Phosphanes, Arsanes, and Stibanes". Angew. Chem. Int. Ed. 43 (8): 938–954. doi:10.1002/anie.200300627. PMID 14966876.