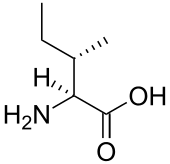
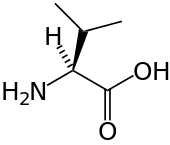
Branched-chain amino acid
A branched-chain amino acid (BCAA) is an amino acid having aliphatic side-chains with a branch (a carbon atom bound to more than two other carbon atoms). Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine and valine.[1]
The BCAAs are among the nine essential amino acids for humans, accounting for 35% of the essential amino acids in muscle proteins and 40% of the preformed amino acids required by mammals.[2]
Research
Dietary BCAA supplementation has been used clinically to aid in the recovery of burn victims. A 2006 paper suggests that the concept of nutrition supplemented with all BCAAs for burns, trauma, and sepsis should be abandoned for a more promising leucine-only-supplemented nutrition that requires further evaluation. [3] Dietary BCAAs are also used in the treatment of some cases of hepatic encephalopathy.[4]
A 2011 study of morbidly obese subjects with type-2 diabetes suggested that the reduction in blood BCAAs resulting from gastric bypass surgery may be associated with improvement in blood sugar regulation. The mechanism remains unknown.[5]
A 2012 study suggests genetic mutations in some autism models lead to altered branched chain amino acid pathways that might be normalized with dietary supplementation.[6]
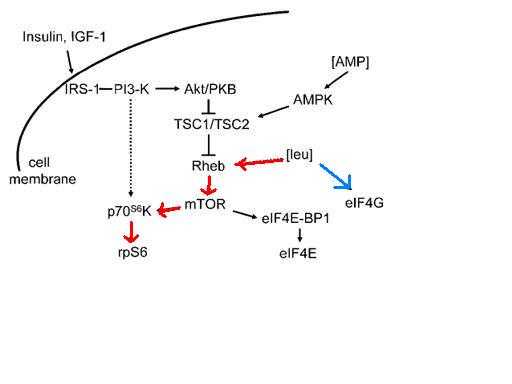
Cota et al (1) demonstrated in 2006 that BCAAs, particularly leucine, also affect the mTOR pathway, signaling two regions of the brain. These signals decrease food intake and increase basic metabolic rate. These results led to a clinical trial by Ordman (3) to develop a nutraceutical method for controlling weight gain in humans. The potential of nutrients to signal changes in metabolism is exemplified by how BCAAs may increase muscle repair, but it is important to be aware how it may also decrease appetite.[7][8][9]
Certain studies suggested a possible link between a high incidence of amyotrophic lateral sclerosis among professional American football players and Italian soccer players, and certain sports supplements including BCAAs.[10] In mouse studies, BCAAs were shown to cause cell hyper-excitability resembling that usually observed in ALS patients. The proposed underlying mechanism is that cell hyper-excitability results in increased calcium absorption by the cell and thus brings about cell death, specifically of neuronal cells which have particularly low calcium buffering capabilities.[10] Yet any link between BCAAs and ALS remains to be fully established. While BCAAs can induce a hyperexcitability similar to the one observed in mice with ALS, current work does not show if a BCAA-enriched diet, given over a prolonged period, actually induces ALS-like symptoms.[10]
Degradation
Degradation of branched-chain amino acids involves the branched-chain alpha-keto acid dehydrogenase complex (BCKDH). A deficiency of this complex leads to a buildup of the branched-chain amino acids (leucine, isoleucine, and valine) and their toxic by-products in the blood and urine, giving the condition the name maple syrup urine disease.
The BCKDH complex converts branched-chain amino acids into Acyl-CoA derivatives, which after subsequent reactions are converted either into acetyl-CoA or succinyl-CoA that enter the citric acid cycle.[11]
Enzymes involved are branched chain aminotransferase and 3-methyl-2-oxobutanoate dehydrogenase.
See also
References
- ↑ Sowers, Strakie. "A Primer On Branched Chain Amino Acids". Huntington College of Health Sciences. Retrieved 22 March 2011.
- ↑ "Exercise Promotes BCAA Catabolism: Effects of BCAA Supplementation on Skeletal Muscle during Exercise". J. Nutr. 134 (6): 1583S–1587S. 2004. Retrieved 22 March 2011.
|coauthors=requires|author=(help) - ↑ "Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis". J. Nutr. 1 Suppl 136 (30): 8S–13S. 2006. Retrieved 22 March 2011.
|coauthors=requires|author=(help) - ↑ "Nutrition in hepatic encephalopathy". Nutr Clin Pract. 25 (3): 257–64. 2010. doi:10.1177/0884533610368712.
|coauthors=requires|author=(help) - ↑ "Differential Metabolic Impact of Gastric Bypass Surgery Versus Dietary Intervention in Obese Diabetic Subjects Despite Identical Weight Loss". Sci Transl Med 3 (80re2). 2011. doi:10.1126/scitranslmed.3002043. Retrieved 27 April 2011.
|coauthors=requires|author=(help) - ↑ Gellar, MD, Barbara (10/01/2012). "A Possibly Treatable Type of Autism". Journal Watch Psychiatry (October).
- ↑ 1) http://www.sciencemag.org/content/312/5775/927.abstract
- ↑ 2) http://chemistry.beloit.edu/Ordman/nutrition/wghtprod.htm
- ↑ 3)http://www.springerlink.com/content/4703748150rj4033/?p=8ca0b6f79ba34e6da6d5ac5419462cf6&pi=0
- ↑ 10.0 10.1 10.2 Manuel, M.; Heckman, C. J. (2011). "Stronger is not always better: Could a bodybuilding dietary supplement lead to ALS?". Experimental Neurology 228 (1): 5–8. doi:10.1016/j.expneurol.2010.12.007. PMC 3049458. PMID 21167830.
- ↑ "Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization". Proc. Nat. Acad. Sci. USA 106 (44): 18745–18750. 2009. doi:10.1073/pnas.0903032106. Retrieved 22 March 2011.
|coauthors=requires|author=(help)
Further reading
- Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E (2004). "Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise". Am. J. Physiol. Endocrinol. Metab. 287 (1): E1–7. doi:10.1152/ajpendo.00430.2003. PMID 14998784.
- Blomstrand E, Eliasson J, Karlsson HK, Köhnke R (2006). "Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise". J. Nutr. 136 (1 Suppl): 269S–73S. PMID 16365096.
- Norton LE, Layman DK (2006). "Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise". J. Nutr. 136 (2): 533S–537S. PMID 16424142.
External links
- Branched-chain amino acids at the US National Library of Medicine Medical Subject Headings (MeSH)
- branched-chain amino acid degradation pathway
| |||||||||||
| |||||||||||||||||||||||||||||||