Borane dimethylsulfide
| Borane dimethylsulfide | ||
|---|---|---|
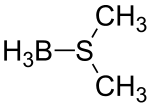 | ||
| Other names BMS, Borane-dimethyl sulfide | ||
| Identifiers | ||
| CAS number | 13292-87-0 | |
| Properties | ||
| Molecular formula | (CH3)2S•BH3 | |
| Molar mass | 75.96 g/mol | |
| Appearance | colorless liquid | |
| Density | 0.801 g/mL | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Borane dimethylsulfide (BMS) is a complexed borane reagent that is used for hydroborations and reductions. The advantages of BMS over other borane reagents, such as borane-tetrahydrofuran, are its increased stability and higher solubility.[1] BMS is commercially available at much higher concentrations than its tetrahydrofuran counterpart (10 M neat) and does not require sodium borohydride as a stabilizer, which could result in undesired side reactions.2 Borane THF uses sodium borohydride to inhibit reductive C-O bond cleavage forming tributyl borate.[2] BMS is soluble in most aprotic solvents.
Preparation and structure
Borane-dimethylsulfide is prepared by absorbing diborane into dimethyl sulfide.[3] It can be purified to by bulb to bulb vacuum transfer. Although a structure of BMS has not been characterized, (pentafluorophenyl)-borane dimethylsulfide (C6F5BH2SMe2), has been examined by X-ray crystallography.[4] The boron center adopts a tetrahedral geometry.
Reactions
Hydroborations
Due to the experimental ease of its use, BMS has become common in hydroboration reactions.[5] In hydroborations with BMS, the dimethylsulfide dissociates in situ, liberating the borane, which rapidly adds to the unsaturated bonds. The resulting organoborane compounds are useful intermediates in organic synthesis. BMS generally performs anti-Markovnikov additions to alkenes and transforms alkynes to the corresponding cis-alkenes.
Reductions
BMS has been commonly employed for the reduction of many functional groups. Reductions of aldehydes, ketones, epoxides, esters, and carboxylic acids give the corresponding alcohols. Lactones are reduced to diols, and nitriles are reduced to amines. Acid chlorides and nitro groups are not reduced by BMS.
Borane diemethylsulfide is one of the most common bulk reducing agents used in the Corey–Itsuno reduction. The dimethyl sulfide ligand attenuates the reactivity of the borane. Activation by the nitrogen of the chiral oxazoborolidine catalyst of the stoichiometric reducing agent allows for asymmetric control of the reagent. In general BMS does not lead to significantly greater enantiomeric selectivites than borane-THF, however its increased stability in the presence of moisture and oxygen makes it the reagent of choice for the reduction.[6]
References
- ↑ Hutchins, Robert O.; Cistone, Frank (1981). "Utility and Applications of Borane Dimethylsulfide in Organic Synthesis. A Review". Organic Preparations and Procedures International 13 (3–4): 225. doi:10.1080/00304948109356130.
- ↑ Kollonitisch, J. (1961). "Reductive Ring Cleavage of Tetrahydrofurans by Diborane". J. Am. Chem. Soc. 83 (6): 1515. doi:10.1021/ja01467a056.
- ↑ Braun, L. M.; Braun, R. A.; Crissman, R.; Opperman, M.; Adams, R. M. (1971). "Dimethyl Sulfide-Borane. A Convenient Hydroborating Reagent". J. Org. Chem. 36 (16): 2388–2389. doi:10.1021/jo00815a047.
- ↑ Fuller, Anna-Marie; Hughes, David L.; Lancaster, Simon J.; White, Callum M. (2010). "Synthesis and Structure of the Dimethyl Sulfide Adducts of Mono- and Bis(pentafluorophenyl)borane". Organometallics 29 (9): 2194. doi:10.1021/om100152v.
- ↑ Atsushi Abiko (1925), "Dicyclohexylboron Trifluoromethylsulfonate", Org. Synth. 79: 103; Coll. Vol. 10: 273
- ↑ Corey, E.J.; Helal, C. J. (1998). "Reduction of Carbonyl Compounds with Chiral Oxazoborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method". Angew. Chem. Int. Ed. 37 (15): 1986–2012. doi:10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z.