Bismuth pentafluoride
| Bismuth pentafluoride | |
|---|---|
 | |
 | |
| Other names bismuth(V) fluoride | |
| Identifiers | |
| CAS number | 7787-62-4 |
| PubChem | 123260 |
| ChemSpider | 21172752 |
| ChEBI | CHEBI:30426 |
| Jmol-3D images | {{#if:[F-].[F-].[F-].[F-].[F-].[BiH3+3]|Image 1 |
| |
| |
| Properties | |
| Molecular formula | BiF5 |
| Molar mass | 303.97 g mol−1 |
| Appearance | long white needles,[1] colourless crystalline solid[2] |
| Density | 5.40 g cm−3[1] |
| Melting point | 151.4 °C,[2] 154.4 °C[1] |
| Boiling point | 230 °C[1][2] |
| Structure | |
| Coordination geometry |
octahedral Bi |
| Hazards | |
| NFPA 704 |
 0
1
0
|
| Related compounds | |
| Other anions | bismuth trichloride, bismuth tribromide, bismuth triiodide, pentamethylbismuth |
| Other cations | phosphorus pentafluoride, arsenic pentafluoride, antimony pentafluoride |
| Related compounds | bismuth trifluoride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Bismuth pentafluoride, BiF5, is a chemical compound of bismuth and fluorine.
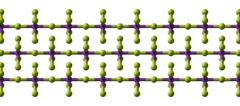
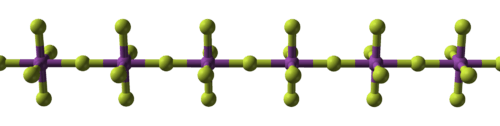
Structure
BiF5 is polymeric and consists of linear chains of trans-bridged corner sharing BiF6 octahedra.[1][3] This is the same structure as α-UF5 and is in contrast to bismuth trifluoride, BiF3, which is ionic and adopts the YF3 structure.[1]
  |   |
Preparation
BiF5 can be prepared by reacting BiF3 with F2 at 500 °C.[2]
- BiF3 + F2 → BiF5
An alternative synthesis uses ClF3 as the fluorinating agent at 350 °C.[4]
- BiF3 + ClF3 → BiF5 + ClF
Reactions
Bismuth pentafluoride is the most reactive of the Group 15 pentafluorides and is an extremely strong fluorinating agent. It reacts vigorously with water to form ozone and oxygen difluoride, and with iodine or sulfur at room temperature. BiF5 fluorinates paraffin oil (hydrocarbons) to fluorocarbons above 50 °C and oxidises UF4 to UF6 at 150 °C. At 180 °C, bismuth pentafluoride fluorinates Br2 to BrF3 and Cl2 to ClF.[1]
BiF5 also reacts with alkali metal fluorides, MF, to form hexafluorobismuthates, M[BiF6], containing the hexafluorobismuthate anion, [BiF6]−.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 561–563. ISBN 0080379419.
- ↑ 2.0 2.1 2.2 2.3 2.4 Holleman, A. F.; Wiberg, E. (2001), Inorganic Chemistry, San Diego: Academic Press, pp. 769–770, ISBN 0-12-352651-5
- ↑ C. Hebecker (1971). "Zur Kristallstruktur von Wismutpentafluorid". Z. anorg. allg. Chem. 384 (2): 111–114. doi:10.1002/zaac.19713840204.
- ↑ A. I. Popov, A. V. Scharabarin, V. F. Sukhoverkhov, N. A. Tchumaevsky (1989). "Synthesis and properties of pentavalent antimony and bismuth fluorides". Z. anorg. allg. Chem. 576 (1): 242–254. doi:10.1002/zaac.19895760128.
| ||||||||||