Beta barrel

A beta barrel is a large beta-sheet that twists and coils to form a closed structure in which the first strand is hydrogen bonded to the last. Beta-strands in beta-barrels are typically arranged in an antiparallel fashion. Barrel structures are commonly found in porins and other proteins that span cell membranes and in proteins that bind hydrophobic ligands in the barrel center, as in lipocalins. Porin-like barrel structures are encoded by as many as 2–3% of the genes in Gram-negative bacteria.[2]
In many cases the strands contain alternating polar and hydrophobic amino acids, so that the hydrophobic residues are oriented into the interior of the barrel to form a hydrophobic core and the polar residues are oriented toward the outside of the barrel on the solvent-exposed surface. Porins and other membrane proteins containing beta barrels reverse this pattern, with hydrophobic residues oriented toward the exterior where they contact the surrounding lipids, and hydrophilic residues oriented toward the interior pore.
All beta-barrels can be classified in terms of two integer parameters: the number of strands in the beta-sheet, n, and the "shear number", S, a measure of the stagger of the strands in the beta-sheet.[3] These two parameters (n and S) are related to the inclination angle of the beta strands relative to the axis of the barrel.[4][5]
Types of beta barrels
Most beta barrels have one of three topologies:
Up-and-down beta barrel
Up-and-down barrels are the simplest barrel topology and consist of a series of beta strands, each of which is hydrogen-bonded to the strands immediately before and after it in the primary sequence.
Greek key
Greek key barrels have some beta strands adjacent in space that are not adjacent in sequence. Beta barrels generally consist of at least one Greek key structural motif linked to a beta hairpin, or two successive Greek keys. N-type and also C-type
Jelly roll
The jelly roll barrel, also known as the Swiss roll, is a complex nonlocal structure in which four pairs of antiparallel beta sheets, only one of which is adjacent in sequence, are "wrapped" in three dimensions to form a barrel shape.
Some functions of beta barrels
Porins
Sixteen- or eighteen-stranded beta barrel structures are common in porins, which function as transporters for ions and small molecules that cannot diffuse across a cellular membrane. Such structures appear in the outer membranes of gram-negative bacteria, chloroplasts, and mitochondria. The central pore of the protein, sometimes known as the eyelet, is lined with charged residues arranged so that the positive and negative charges appear on opposite sides of the pore. A long loop between two beta sheets partially occludes the central channel; the exact size and conformation of the loop helps in discriminating between molecules passing through the transporter.
Preprotein Translocases
Beta barrels also function within endosymbiont derived organelles such as mitochondria and chloroplasts to transport proteins.[6] Within the mitochondrion two complexes exist with beta barrels serving as the pore forming subunit, Tom40 of the Translocase of the outer membrane, and Sam50 of the Sorting and assembly machinery. The chloroplast also has functionally similar beta barrel containing complexes, the best characterised of which is Toc75 of the TOC complex (Translocon at the outer envelope membrane of chloroplasts).
Lipocalins
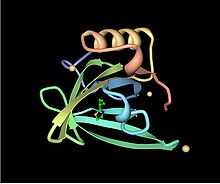
Lipocalins are typically eight-stranded beta barrel proteins that are often secreted into the extracellular environment. Their most distinctive feature is their ability to bind and transport small hydrophobic molecules in a beta barrel calyx. Examples of the family include retinol binding proteins (RBPs) and major urinary proteins (Mups). RBP binds and transports retinol (vitamin A), while Mups bind a number of small, organic pheromones, including 2-sec-butyl-4,5-dihydrothiazole (abbreviated as SBT or DHT), 6-hydroxy-6-methyl-3-heptanone (HMH) and 2,3 dihydro-exo-brevicomin (DHB).[7][8][9]
Shear number
A piece of paper can be formed into a cylinder by bringing opposite sides together. The two edges come together to form a line. Shear can be created by sliding the two edges parallel to that line. Likewise, a beta barrel can be formed by bringing the edges of a beta sheet together to form a cylinder. If those edges are displaced, then shear will be created.
A similar definition of shear is found in geology, where shear refers to a displacement within rock perpendicular to the surface of the rock. In physics, the amount of displacement is referred to as shear strain, which has units of length. Shear number is a measure of shear strain in which the displacement is measured in units of "amino acid residues".
The determination of shear number requires the assumption that each amino acid in one strand of a beta sheet is adjacent to just one amino acid in the neighboring strand. (This assumption may not hold if, for example, a beta bulge is present. [10] ) To illustrate, S will be calculated for green fluorescent protein. This protein was chosen because the beta barrel contains both parallel and antiparallel strands. The particular example used, PDB 1RRX, is one of the few structures of this protein that is not obtained from a mutant protein.
- Green fluorescent protein 1RRX
-

Eleven strands make up the beta barrel.
-

The chain is colored according to position in the chain, from blue (the N terminus) to red (the C terminus).
From the last figure, the order of strands in the barrel is found to be: 1 6 5 4 9 8 7 10 11 3 2.
To determine which amino acid residues are adjacent in the beta strands, the location of hydrogen bonds is determined. The figure below shows the calculated positions of hydrogen bonds. The residues are labeled with a residue number and a one-letter amino acid code (the label is placed near the alpha carbon). Only the backbone atoms of the beta barrel are shown, and, of that, only the front slab is shown. It appears that, for example, residues 31 G, on strand 2, 16 V on strand 1, and 121 N on strand 6 are adjacent.

- The other three slabs
-

Slab 2, obtained by rotating the protein by 90°.
-

Slab 3, obtained by rotating an additional 90°.
-

Slab 4, obtained by rotating an additional 90°.
The data from the figures is collected in the table, below. Each column contains the residues in one strand. Strand 1 is repeated in the last column. The arrows indicate the hydrogen bonds that were identified in the figures. The relative direction of each strand is indicated by the "+" and "-" at the bottom of the table. Except for strands 1 and 6, all strands are antiparallel. The parallel interaction between strands 1 and 6 accounts for the different appearance of the hydrogen bonding pattern. (Some arrows are missing because not all of the hydrogen bonds expected were identified. Also, some residues, such as 182 ?, contain a question mark; this indicates the presence of a non-standard amino acid.) The side chains that point to the outside of the barrel are in bold.

If no shear were present in this barrel, then residue 12 V, say, in strand 1 should end up in the last strand at the same level as it started at. However, because of shear, 12 V is not at the same level: it is 14 residues higher than it started at, so its shear number, S, is 14.
Dynamical features
Beta-barrels in proteins may carry out low-frequency breathing-like motion as observed by the Raman spectroscopy [11] and analyzed with the quasi-continuum model. [12] For more about the low-frequency collective motions in biomacromolecules and its biological function, see low-frequency collective motion in proteins and DNA.
References
- ↑ Böcskei Z, Groom CR, Flower DR, et al. (November 1992). "Pheromone binding to two rodent urinary proteins revealed by X-ray crystallography". Nature 360 (6400): 186–8. doi:10.1038/360186a0. PMID 1279439.
- ↑ Wimley, WC. (2003). "The versatile beta-barrel membrane protein". Curr Opin Struct Biol 13 (4): 404–11. doi:10.1016/S0959-440X(03)00099-X. PMID 12948769.
- ↑ Murzin A, Lesk A, Chothia C (1994). "Principles determining the structure of beta-sheet barrels in proteins. I. A theoretical analysis". J Mol Biol 236 (5): 1369–81. doi:10.1016/0022-2836(94)90064-7. PMID 8126726.
- ↑ Murzin A, Lesk A, Chothia C (1994). "Principles determining the structure of beta-sheet barrels in proteins. II. The observed structures". J Mol Biol 236 (5): 1382–400. doi:10.1016/0022-2836(94)90065-5. PMID 8126727.
- ↑ Liu, WM. (1998). "Shear numbers of protein beta-barrels: definition refinements and statistics". J Mol Biol 275 (4): 541–5. doi:10.1006/jmbi.1997.1501. PMID 9466929.
- ↑ Schleiff, Enrico; Soll, Jürgen (1 November 2005). "Membrane protein insertion: mixing eukaryotic and prokaryotic concepts". EMBO Reports 6 (11): 1023–1027. doi:10.1038/sj.embor.7400563. PMID 16264426.
- ↑ Halpern M, Martínez-Marcos A (June 2003). "Structure and function of the vomeronasal system: an update". Progress in Neurobiology 70 (3): 245–318. doi:10.1016/S0301-0082(03)00103-5. PMID 12951145.
- ↑ Timm DE, Baker LJ, Mueller H, Zidek L, Novotny MV (May 2001). "Structural basis of pheromone binding to mouse major urinary protein (MUP-I)". Protein Science 10 (5): 997–1004. doi:10.1110/ps.52201. PMC 2374202. PMID 11316880.
- ↑ Armstrong SD, Robertson DH, Cheetham SA, Hurst JL, Beynon RJ (October 2005). "Structural and functional differences in isoforms of mouse major urinary proteins: a male-specific protein that preferentially binds a male pheromone". The Biochemical Journal 391 (Pt 2): 343–50. doi:10.1042/BJ20050404. PMC 1276933. PMID 15934926.
- ↑ Nagano, N.; Hutchinson, E. G.; Thornton, J. M. (1999). "Barrel structures in proteins: Automatic identification and classification including a sequence analysis of TIM barrels". Protein Science (Wiley-Blackwell) 8 (10): 2072–2084. doi:10.1110/ps.8.10.2072. PMC 2144152. PMID 10548053. Retrieved 2008-12-31.
- ↑ Painter, P.C., Mosher, L.E. and Rhoads, C. (1982) Low-frequency modes in the Raman spectra of proteins" Biopolymers 21, 1469-1472.
- ↑ Kuo-Chen Chou (1985) Low-frequency motions in protein molecules: beta-sheet and beta-barrel" Biophysical Journal 48, 289-297.
Further reading
- Branden C, Tooze J. (1999). Introduction to Protein Structure 2nd ed. Garland Publishing: New York, NY. ISBN 0-8153-2304-2.
External links
- Explanation of all-beta topologies: "orthogonal beta-sandwiches" are beta-barrels (as defined in this article); "aligned" beta-sandwiches" correspond to beta-sandwich folds in SCOP classification.
- all-beta folds in SCOP database (folds 54 to 100 are water-soluble beta-barrels).
- General classification and images of protein structures from Jane Richardson lab
- Images and examples of transmembrane beta-barrels
- Stockholm Bioinformatics Center review of transmembrane proteins
- The Lipocalin Website
| |||||||||||||||||||||||
