Beta-glucan


β-Glucans (beta-glucans) are polysaccharides of D-glucose monomers linked by β-glycosidic bonds. β-glucans are a diverse group of molecules that can vary with respect to molecular mass, solubility, viscosity, and three-dimensional configuration. They occur most commonly as cellulose in plants, the bran of cereal grains, the cell wall of baker's yeast, certain fungi, mushrooms and bacteria. Some forms of beta glucans are useful in human nutrition as texturing agents and as soluble fiber supplements, but can be problematic in the process of brewing.
Oat is a rich source of the water-soluble fibre (1,3/1,4) β-glucan, and its effects on health have been extensively studied the last 30 years. Oat β-glucans are the only dietary fiber currently recognized by the European Food Safety Authority (EFSA) to be able to reduce a disease risk.[1] Oat β-glucans can be highly concentrated in different types of oat brans.
"Barley has more beta glucan fiber than any other grain" claims a report on DiabetesHealth website ; 11 sources are listed.[2]
Yeast and medicinal mushroom derived β-glucans are notable for their ability to modulate the immune system. One study has shown that insoluble (1,3/1,6) β-glucan, has greater biological activity than that of its soluble (1,3/1,4) β-glucan counterparts.[3] The differences between β-glucan linkages and chemical structure are significant in regards to solubility, mode of action, and overall biological activity.
Overview

β-Glucans are polysaccharides that contain only glucose as structural components, and are linked with β-glycosidic bonds.
Glycosidic bonds are etheric oxygen bridges that link the monosaccharide units in a polysaccharide, and they are designated by a pair of numbers to indicate which carbons in each of the monosaccharide units are linked (according to the standard numbering for simple monosaccharides). When a glycosidic bond involves the number 1 carbon of an aldose monosaccharide (or the number 2 carbon of a ketose monosaccharide), a second designation is needed to indicate the spatial orientation of the etheric oxygen linkage at that carbon (the anomeric carbon). An "α-" (alpha) indicates the etheric oxygen linkage attaches to the anomeric carbon below the ring, and a "β-" (beta) indicates the etheric oxygen linkage attaches to the anomeric carbon above the ring (in the standard Haworth projection). The orientation of the etheric linkage at any of the other carbons is predetermined and fixed by the identity of the monosaccharide and the position, and no further descriptor is required for the glycosidic bond.
Thus, the designation of β(1-3) for a glycosidic linkage indicates that the etheric oxygen bridge between two consecutive monosaccharide units of the polysaccharide connects the number 1 carbon of the first unit to the number 3 carbon of the second unit, and that etheric oxygen bridge attaches to carbon 1 of the first unit from above the ring.
Likewise, the designation of β(1-6) for a glycosidic linkage indicates that the etheric oxygen bridge between two consecutive monosaccharide units of the polysaccharide connects the number 1 carbon of the first unit to the number 6 carbon of the second unit, and that etheric oxygen bridge attaches to carbon 1 of the first unit from above the ring.
Beta-glucan chemistry
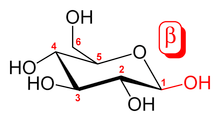
By definition, beta-glucans are chains of D-glucose polysaccharides, linked by beta-type glycosidic bonds. These six-sided D-glucose rings can be connected to one another, on a variety of positions on the D-glucose ring structure. Some β-glucan compounds are continual repeats of D-glucose attached at a specific position.
However, β-glucans can be more diverse than molecules like starch. For instance, a β-glucan molecule can be composed of repeating D-glucose units linked with β-glycosidic bonds at one position like starch, but have branching glucose side-chains attached to other positions on the main D-glucose chain. These smaller side-chains can branch off the β-glucan “backbone" (in the case of starch, the backbone would be D-glucose chains linked at the (1,4) position) at other positions like that of the 3 and 6 position. In addition, these side-chains can be attached to other types of molecules, like proteins. An example of a β-glucan that has proteins attached to it is Polysaccharide-K.
The most active forms of β-glucans are those comprising D-glucose units with (1,3) links and with side-chains of D-glucose attached at the (1,6) position. These are referred to as β-1,3/1,6 glucan. Some researchers have suggested that it is the frequency, location, and length of the side-chains rather than the backbone of β-glucans that determine their immune system activity. Another variable is the fact that some of these compounds exist as single-strand chains, while the backbones of other β(1,3)-glucans exist as double- or triple-stranded helix chains. In some cases, proteins linked to the β(1,3)-glucan backbone may also be involved in providing therapeutic activity. Although these compounds can potentially enhance immune function, it must be emphasized that this research is in its infancy. In addition, there are differing opinions on which molecular weight, shape, structure, and source of β(1,3)-glucans provide the greatest biological activity.
β-glucan sources in nature

One of the most common sources of β(1,3)D-glucan for supplement use is derived from the cell wall of baker’s yeast (Saccharomyces cerevisiae). However, β(1,3)(1,4)-glucans are also extracted from the bran of some grains, such as oats and barley, and to a much lesser degree in rye and wheat. The β(1,3)D-glucans from yeast are often insoluble. Those extracted from grains tend to be both soluble and insoluble. Other sources include some types of seaweed,[4] and various species of mushrooms, such as reishi, shiitake, Chaga and maitake.[5]
β-Glucan and the immune system
β-Glucans are known as "biological response modifiers" because of their ability to activate the immune system.[6] Immunologists have discovered that receptors on the surface of innate immune cells called dectin-1 and complement receptor 3 (CR3 or CD11b/CD18) are responsible for binding to β-glucans, allowing the immune cells to recognize them as "non-self".[7][8]
β-glucan and blood cholesterol
Several health claims requests were submitted to the EFSA NDA Panel (Dietetic Products, Nutrition and Allergies), related to the role of β-glucans in maintenance of normal blood cholesterol concentrations and maintenance or achievement of a normal body weight. In July 2009, the Scientific Committee issued the following statements:[9]
- On the basis of the data available, the Panel concludes that a cause-and-effect relationship has been established between the consumption of beta-glucans and the "reduction of blood cholesterol concentrations."
- The following wording reflects the scientific evidence: "Regular consumption of beta-glucans contributes to maintenance of normal blood cholesterol concentrations." In order to bear the claim, foods should provide at least 3 g/d of beta-glucans from oats, oat bran, barley, barley bran, or mixtures of non-processed or minimally processed beta-glucans in one or more servings. The target population is adults with normal or mildly elevated blood cholesterol concentrations.
- On the basis of the data available the Panel concludes that a cause-and-effect relationship has not been established between the consumption of beta-glucans and the maintenance or achievement of a normal body weight.
In November 2011, the EU Commission published its decision in favour of oat beta-glucans with respect to Article 14 of the EC Regulation on the labelling of foodstuffs with nutrition and health claim statements permitting oat beta-glucan to be described as beneficial to health. Following the opinion of the Panel on Dietetic Products, Nutrition and Allergies (NDA) the EFSA and the Regulation (EU) no. 1160/2011 of the Commission, foodstuffs through which 3 g/day of oat beta-glucan are consumed (1 g of oat beta-glucan per portion) are allowed to display the following health claim: «Oat beta-glucan reduces the cholesterol level in the blood. The lowering of the blood cholesterol level can reduce the risk of coronary heart disease.»[10]
Research
Tumoricidal effects
The tumoricidal properties of β-glucans have been studied in several in vitro and in vivo animal models.[11][12][13] In a mouse model study, β-1,3 glucan in conjunction with interferon gamma inhibited tumors and liver metastasis.[14] In some studies, β-1,3 glucans enhanced the effects of chemotherapy. In a mouse carcinoma model, β-1,3 glucans did not reduce tumor incidence, but were associated with reduced mortality in combination with cyclophosphamide.[15] In human patients with advanced gastric or colorectal cancer, the administration of β-1,3 glucans derived from shiitake mushrooms, in conjunction with chemotherapy, resulted in prolonged survival times.[16]
Prevention of infection
Alpha-Beta Technologies conducted a series of human clinical trials in the 1990s to evaluate the impact of β-glucan therapy for controlling infections in high-risk surgical patients.[17] In the initial trial, 34 patients were randomly (double-blind, placebo-controlled) assigned to treatment or placebo groups. Patients having received the PGG-glucan had significantly fewer infectious complications than the placebo group (1.4 infections per infected patient for PGG-glucan group vs. 3.4 infections per infected patient for the placebo group). Additional data from the clinical trial revealed intravenous antibiotic use was decreased, and stays in the intensive-care unit were shorter for the patients receiving PGG-glucan vs. patients receiving the placebo.
A subsequent human clinical trial[18] studied the effect of β-glucan on the incidence of infection in high-risk surgical patients. A total of 67 patients were randomized to treatment with a placebo or a dose of 0.1, 0.5, 1.0 or 2.0 mg PGG-glucan per kilogram of body weight. Serious infections occurred in four patients having received the placebo, three patients having received the low dose (0.1 mg/kg) of PGG-glucan, and one patient having received the highest dose of 2.0 mg/kg.
The results of a phase III human clinical trial showed that PGG-glucan therapy reduced serious postoperative infections by 39% after high-risk noncolorectal operations.[19] This study was conducted in patients already at high risk because of the type of surgery and were more susceptible to infections and other complications.
A study conducted by the Canadian Department of Defense showed that orally administered yeast β-glucan given with or without antibiotics protected mice against anthrax infection.[20] A dose of antibiotics along with oral whole glucan particles (2 mg/kg body weight or 20 mg/kg body weight) for eight days prior to infection with Bacillus anthracis protected mice against anthrax infection over the 10-day postexposure test period. Mice treated with the antibiotic alone did not survive.
A second experiment was conducted to investigate the effect of yeast β-glucan orally consumed after exposure of mice to B. anthracis. The results were similar to the previous experiment, with an 80-90% survival rate for mice treated with β-glucan, but only 30% for the control group after 10 days of exposure.
Early research by Onderdonk et al.[21] investigated the ability of yeast b-glucan to reduce septic infections using in vivo models. They found that mice challenged with E. coli or S. aureus bacteria were protected against septic infections when they were injected with PGG-glucan 4–6 hr prior to infection. Preventative dosing of yeast β-glucan prior to infection with S. aureus prevented sepsis in a guinea pig model.[22] Research has been conducted in animals on the use of yeast β-glucans for the treatment and prophylaxis of bacterial sepsis[21][22][23] and protection against oxidative organ injury.[24]
In a prospective, randomized, double-blind study, 38 trauma patients received a soluble, yeast-derived glucan intravenously for seven days or placebo. The total mortality rate was significantly less in the glucan group (0% vs. 29%), with also a decrease in septic morbidity (9.5% vs. 49%).[25]
Yeast-derived beta-glucan significantly enhanced phagocytic activity in an experimental mouse model of sepsis induced by Candida albicans and midline laparotomy. Mice not operated on, on glucan, had a 100% survival vs. 73% in the surgical group.[26]
Radiation exposure
Specific hematopoietic activity was first demonstrated with β-glucan in the mid-1980s in an analogous manner as granulocyte monocyte–colony-stimulating factor (GM-CSF).[27] Research was carried out initially with particulate β-glucan and later with soluble β-glucans, all of which administered intravenously (IV) to mice.[28][29][30] Mice exposed to 500-900 cGy (500-900 mrads) of gamma radiation exhibited a significantly enhanced recovery of blood leukocyte, platelet and red blood cell counts when given IV β-glucan.[31] Other reports showed that β-glucan could reverse the myelosuppression produced with chemotherapeutic drugs such as fluorouracil,[19] carboplatinum, or cyclophosphamide.[32] Moreover, the anti-infective activity of β-glucan combined with its hematopoiesis-stimulating activity resulted in enhanced survival of mice receiving a lethal dose of 900-1200 cGy of radiation.[17] In vitro studies showed β-glucan could enhance granulocyte and megakaryocyte colony formation by hematopoietic stem progenitor cells when used in combination with GM-CSF and interleukin-3, respectively.[33]
Original studies delivered glucan almost entirely by injection. Later, numerous studies tried to evaluate the possibility that glucan can be delivered orally without compromising its biological activities,[8][34][35][36] opening the oral route of administration as a more pleasant alternative. Oral beta-glucan had hematopoietic effects analogous to beta-glucan administered by IV, and orally administered whole glucan particulate functions to accelerate hematopoiesis following irradiation in an analogous manner as IV-administered β-glucan.[37] The mechanisms of the glucan transfer through the gastrointestinal tract were reported.[8] Oral β-glucan stimulates hematopoiesis in radiation-treated mice.[8][38]
Allergic rhinitis
This disease is caused by an IgE-mediated allergic inflammation of the nasal mucosa. Orally administered yeast-glucan decreased levels of IL-4 and IL-5 cytokines responsible for the clinical manifestation of this disease, while increased the levels of IL-12.[39]
Arthritis
A study employing paramagnetic resonance spectroscopy indicated that yeast-derived beta-glucan could reduce free-radical formation in vitro and reduce the plasma levels of protein carbonyls in a rodent arthritis model.[40]
Additional applications
Consuming certain cereals (barley, oats) and edible mushrooms decreases the levels of serum cholesterol and liver low-density lipoproteins, leading to lowering of atherosclerosis and cardiovascular disease hazards; this is also mediated by β-glucans.[41] These cereals, mushrooms, and yeast facilitate bowel motility and can be used in amelioration of intestinal problems, particularly obstipation.[42][43] Indigestible β-glucans, forming a remarkable portion of these materials, are also able to modulate mucosal immunity of the intestinal tract.[44]
β-Glucan absorption
For maximal absorption, oral β(1,3)-D-glucan should be taken on an empty stomach. It is reported that enterocytes facilitate the transportation of β(1,3)-glucans and similar compounds across the intestinal cell wall into the lymph, where they begin to interact with macrophages to activate immune function.[45] Radiolabeled studies have verified that both small and large fragments of β-glucans are found in the serum, which indicates that they are absorbed from the intestinal tract.[46] M cells within the Peyer’s patches physically transport the insoluble whole glucan particles into the gut-associated lymphoid tissue.[34]
Role in diagnostics
β-D-Glucan forms part of the cell wall of certain medically important fungi, especially Aspergillus and Agaricus species. An assay to detect its presence in blood is marketed as a means of diagnosing invasive fungal infection in patients.[47][48][49]
False positives may occur because of fungal contaminants in the antibiotics amoxicillin-clavulanate,[50] and piperacillin/tazobactam. False positives can also occur with contamination of clinical specimens with the bacteria Streptococcus pneumoniae, Pseudomonas aeruginosa, and Alcaligenes faecalis, which also produce (1→3)β-D-glucan.[51]
See also
- Medicinal mushrooms
Notes
- ↑ European Commission. "Regulation 1160/2011". on the authorisation and refusal of authorisation of certain health claims made on foods and referring to the reduction of disease risk. Retrieved 14 November 2011.
- ↑ Mohiuddin, Safia Fatima. "An Inside View of Barley Beta Glucan, Feb 5, 2012". DiabetesHealth. Retrieved 28 November 2012.
- ↑ Ooi VE, Liu F (July 2000). "Immunomodulation and anti-cancer activity of polysaccharide-protein complexes". Curr. Med. Chem. 7 (7): 715–29. PMID 10702635.
- ↑ Teas, J (1983). "The dietary intake of Laminarin, a brown seaweed, and breast cancer prevention". Nutrition and cancer (Lawrence Erlbaum Associates) 4 (3): 217–222. doi:10.1080/01635588209513760. ISSN 0163-5581. PMID 6302638.
- ↑ Wasser, SP; Weis AL (1999). "Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective". Critical reviews in immunology (United States: Begell House) 19 (1): 65–96. ISSN 1040-8401. PMID 9987601.
- ↑ Miura, NN; Ohno N, Aketagawa J, Tamura H, Tanaka S, Yadomae T (January 1996). "Blood clearance of (1-->3)-beta-D-glucan in MRL lpr/lpr mice". FEMS immunology and medical microbiology (England: Blackwell Publishing) 13 (1): 51–57. ISSN 0928-8244. PMID 8821398.
- ↑ Brown, GD; Gordon, S (Sep 6, 2001). "Immune recognition. A new receptor for beta-glucans.". Nature 413 (6851): 36–7. doi:10.1038/35092620. PMID 11544516.
- ↑ 8.0 8.1 8.2 8.3 Vetvicka, V; Dvorak B, Vetvickova J, Richter J, Krizan J, Sima P, Yvin JC (2007-03-10). "Orally administered marine (1-->3)-beta-D-glucan Phycarine stimulates both humoral and cellular immunity". International journal of biological macromolecules (England: Butterworth-Heinemann) 40 (4): 291–298. doi:10.1016/j.ijbiomac.2006.08.009. PMID 16978690.
- ↑ Bresson, Jean-Louis; Albert Flynn, Marina Heinonen, Karin Hulshof, Hannu Korhonen, Pagona Lagiou, Martinus Løvik, Rosangela Marchelli, Ambroise Martin, Bevan Moseley, Hildegard Przyrembel, Seppo Salminen, Sean (J.J.) Strain, Stephan Strobel, Inge Tetens, Henk van den Berg, Hendrik van Loveren and Hans Verhagen (2009). "Scientific Opinion on the substantiation of health claims related to beta glucans and maintenance of normal blood cholesterol concentrations (ID 754, 755, 757, 801, 1465, 2934) and maintenance or achievement of a normal body weight (ID 820, 823) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal 7 (9): 1254. doi:10.2903/j.efsa.2009.1254. Retrieved 2 March 2011.
- ↑ European Commission. "Regulation 1160/2011". on the authorisation and refusal of authorisation of certain health claims made on foods and referring to the reduction of disease risk. Official Journal of the European Union. Retrieved 14 November 2011.
- ↑ DiLuzio, NR; Williams DL, McNamee RB, Malshet VG (1980). "Comparative evaluation of the tumor inhibitory and antibacterial activity of solubilized and particulate glucan". Recent results in cancer research. Fortschritte der Krebsforschung. Progrès dans les recherches sur le cancer (Germany: Springer Verlag) 75: 165–172. ISSN 0080-0015. PMID 7232829.
- ↑ Morikawa, K; Takeda R, Yamazaki M, Mizuno D (April 1985). "Induction of tumoricidal activity of polymorphonuclear leukocytes by a linear beta-1,3-D-glucan and other immunomodulators in murine cells". Cancer Research (United States: American Association for Cancer Research) 45 (4): 1496–1501. ISSN 0008-5472. PMID 3156669.
- ↑ Mansell, PW; Ichinose H, Reed RJ, Krementz ET, McNamee R, Di Luzio NR (March 1975). "Macrophage-mediated destruction of human malignant cells in vivo". Journal of the National Cancer Institute (United States: Oxford University Press) 54 (3): 571–580. ISSN 0027-8874. PMID 1123850.
- ↑ Sveinbjørnsson, B; Rushfeldt C, Seljelid R, Smedsrød B (May 1998). "Inhibition of establishment and growth of mouse liver metastases after treatment with interferon gamma and beta-1,3-D-glucan". Hepatology (Baltimore, Md.) (United States: Wiley) 27 (5): 1241–1248. doi:10.1002/hep.510270509. PMID 9581677.
- ↑ Thompson, IM; Spence CR, Lamm DL, DiLuzio NR (November 1987). "Immunochemotherapy of bladder carcinoma with glucan and cyclophosphamide". The American journal of the medical sciences (United States: Lippincott Williams & Wilkins) 294 (5): 294–300. doi:10.1097/00000441-198711000-00002. PMID 3425579.
- ↑ Wakui, A; Kasai M, Konno K, Abe R, Kanamaru R, Takahashi K, Nakai Y, Yoshida Y, Koie H, Masuda H, et al. (April 1986). "Randomized study of lentinan on patients with advanced gastric and colorectal cancer. Tohoku Lentinan Study Group". Gan to kagaku ryoho. Cancer & chemotherapy (in Japanese) (Japan: Gan To Kagaku Ryohosha) 13 (4 pt 1): 1050–1059. ISSN 0385-0684. PMID 3083785.
- ↑ 17.0 17.1 Babineau, TJ; Marcello P, Swails W, Kenler A, Bistrian B, Forse RA (November 1994). "Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients". Annals of surgery (United States: Lippincott Williams & Wilkins) 220 (5): 601–609. doi:10.1097/00000658-199411000-00002. PMC 1234447. PMID 7979607.
- ↑ Babineau, TJ; Hackford A, Kenler A, Bistrian B, Forse RA, Fairchild PG, Heard S, Keroack M, Caushaj P, Benotti P (November 1994). "A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients". Archives of surgery (Chicago, Ill. : 1960) (United States: American Medical Association) 129 (11): 1204–1210. ISSN 0004-0010. PMID 7979954.
- ↑ 19.0 19.1 Dellinger, EP; Babineau TJ, Bleicher P, Kaiser AB, Seibert GB, Postier RG, Vogel SB, Norman J, Kaufman D, Galandiuk S, Condon RE (September 1999). "Effect of PGG-glucan on the rate of serious postoperative infection or death observed after high-risk gastrointestinal operations. Betafectin Gastrointestinal Study Group". Archives of surgery (Chicago, Ill. : 1960) (United States: American Medical Association) 134 (9): 977–983. doi:10.1001/archsurg.134.9.977. PMID 10487593.
- ↑ Vetvicka, V; Terayama K, Mandeville R, Brousseau P, Kournikakis B, Ostroff G (2002). "Pilot Study: Orally-Administered Yeast β1,3-glucan Prophylactically Protects Against Anthrax Infection and Cancer in Mice" (PDF). Journal of the American Nutraceutical Association (Birmingham, AL : The Association) 5 (2): 5–9. ISSN 1521-4524. Archived from the original on 2011-07-24.
- ↑ 21.0 21.1 Onderdonk, AB; Cisneros RL, Hinkson P, Ostroff G (April 1992). "Anti-infective effect of poly-beta 1-6-glucotriosyl-beta 1-3-glucopyranose glucan in vivo". Infection and immunity (United States: American Society for Microbiology) 60 (4): 1642–1647. ISSN 0019-9567. PMC 257041. PMID 1548086.
- ↑ 22.0 22.1 Kernodle, DS; Gates H, Kaiser AB (March 1998). "Prophylactic Anti-Infective Activity of Poly-1-6-β-d-Glucopyranosyl-1-3-β-d-Glucopyranose Glucan in a Guinea Pig Model of Staphylococcal Wound Infection". Antimicrobial agents and chemotherapy (United States: American Society for Microbiology) 42 (3): 545–549. ISSN 0066-4804. PMC 105496. PMID 9517930.
- ↑ Tzianabos, AO; Gibson FC 3rd, Cisneros RL, Kasper DL (July 1998). "Protection against experimental intraabdominal sepsis by two polysaccharide immunomodulators". The Journal of infectious diseases (United States: University of Chicago Press) 178 (1): 200–206. ISSN 0022-1899. PMID 9652441.
- ↑ Sener, G; Toklu H, Ercan F, Erkanli G (August 2005). "Protective effect of beta-glucan against oxidative organ injury in a rat model of sepsis". International immunopharmacology (Netherlands: Elsevier Science) 5 (9): 1387–1396. doi:10.1016/j.intimp.2005.03.007. PMID 15953565.
- ↑ Browder, W; Williams D, Pretus H, Olivero G, Enrichens F, Mao P, Franchello A (May 1990). "Beneficial effect of enhanced macrophage function in the trauma patient". Annals of surgery (United States: Lippincott Williams & Wilkins) 211 (5): 605–612; discussion 612–613. ISSN 0003-4932. PMC 1358234. PMID 2111126.
- ↑ Browder, IW; Williams DL, Kitahama A, Di Luzio NR (1984). "Modification of post-operative C. albicans sepsis by glucan immunostimulation". International journal of immunopharmacology (England: Elsevier Science) 6 (1): 19–26. doi:10.1016/0192-0561(84)90030-4. PMID 6724765.
- ↑ Patchen, ML; MacVittie TJ (April 1983). "Dose-dependent responses of murine pluripotent stem cells and myeloid and erythroid progenitor cells following administration of the immunomodulating agent glucan". Immunopharmacology (Netherlands: Elsevier/North-Holland) 5 (4): 303–313. doi:10.1016/0162-3109(83)90046-2. PMID 6853144.
- ↑ Patchen, ML; DiLuzio NR, Jacques P, MacVittie TJ (December 1984). "Soluble polyglycans enhance recovery from cobalt-60--induced hemopoietic injury". Journal of biological response modifiers (United States: Raven Press) 3 (6): 627–633. ISSN 0732-6580. PMID 6512563.
- ↑ Patchen, ML; MacVittie TJ, Wathen LM (1984-11-15). "Effects of pre- and post-irradiation glucan treatment on pluripotent stem cells, granulocyte, macrophage and erythroid progenitor cells, and hemopoietic stromal cells". Experientia (Switzerland: Birkhäuser Verlag) 40 (11): 1240–1244. doi:10.1007/BF01946654. PMID 6500009.
- ↑ Petruczenko, A (May–June 1984). "Glucan effect on the survival of mice after radiation exposure". Acta physiologica Polonica (Poland: Panstwowy Zaklad Wydawnictw Lekarskich) 35 (3): 231–236. ISSN 0044-6033. PMID 6537716.
- ↑ Patchen, ML; MacVittie TJ (February 1986). "Comparative effects of soluble and particulate glucans on survival in irradiated mice". Journal of biological response modifiers (United States: Raven Press) 5 (1): 45–60. ISSN 0732-6580. PMID 3958754.
- ↑ Patchen, ML; Vaudrain T, Correira H, Martin T, Reese D (December 1998). "In vitro and in vivo hematopoietic activities of Betafectin PGG-glucan". Experimental hematology (Netherlands: Elsevier Science) 26 (13): 1247–1254. ISSN 0301-472X. PMID 9845381.
- ↑ Turnbull, JL; Patchen ML, Scadden DT (1999). "The polysaccharide, PGG-glucan, enhances human myelopoiesis by direct action independent of and additive to early-acting cytokines". Acta haematologica (Switzerland: Karger) 102 (2): 66–71. doi:10.1159/000040972. PMID 10529508.
- ↑ 34.0 34.1 Hong, F; Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD (2004-07-15). "Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models". Journal of immunology (Baltimore, Md. : 1950) (United States: American Association of Immunologists) 173 (2): 797–806. ISSN 0022-1767. PMID 15240666.
- ↑ Tomoda, M; Ohara N, Shimizu N, Gonda R (March 1994). "Characterization of a novel glucan, which exhibits reticuloendothelial system-potentiating and anti-complementary activities, from the rhizome of Cnidium officinale". Chemical & pharmaceutical bulletin (Japan: Pharmaceutical Society of Japan) 42 (3): 630–633. ISSN 0009-2363. PMID 8004712.
- ↑ Mucksová, J; Babícek K, Pospísil M (2001). "Particulate 1,3-beta-D-glucan, carboxymethylglucan and sulfoethylglucan--influence of their oral or intraperitoneal administration on immunological respondence of mice". Folia microbiologica (Czech Republic: Slovak Academy Of Sciences) 46 (6): 559–563. doi:10.1007/BF02818003. PMID 11898349.
- ↑ Allendorf et al., Oral WGP Beta Glucan Treatment Accelerates Myeloid Recovery after Radiation Exposure. Presented at BTR 2003
- ↑ Cramer, DE; Allendorf DJ, Baran JT, Hansen R, Marroquin J, Li B, Ratajczak J, Ratajczak MZ, Yan J (2006-01-15). "β-Glucan enhances complement-mediated hematopoietic recovery after bone marrow injury". Blood (United States: American Society of Hematology) 107 (2): 835–840. doi:10.1182/blood-2005-07-2705. PMC 1895628. PMID 16179370.
- ↑ Kirmaz, C; Bayrak P, Yilmaz O, Yuksel H. (June 2005). "Effects of glucan treatment on the Th1/Th2 balance in patients with allergic rhinitis: a double-blind placebo-controlled study". European cytokine network (France: John Libbey Eurotext) 16 (2): 128–134. ISSN 1148-5493. PMID 15941684.
- ↑ Kogan, G; Stasko A, Bauerova K, Polovka M, Soltes L, Brezova V, Navarova J (2005-07-04). "Antioxidant properties of yeast (1→3)-β-D-glucan studied by electron paramagnetic resonance spectroscopy and its activity in the adjuvant arthritis". Carbohydrate Polymers (Elsevier) 61 (1): 18–28. doi:10.1016/j.carbpol.2005.02.010.
- ↑ Keogh, GF; Cooper GJ, Mulvey TB, McArdle BH, Coles GD, Monro JA, Poppitt SD (October 2003). "Randomized controlled crossover study of the effect of a highly beta-glucan-enriched barley on cardiovascular disease risk factors in mildly hypercholesterolemic men". The American journal of clinical nutrition (United States: American Society of Clinical Nutrition) 78 (4): 711–718. ISSN 0002-9165. PMID 14522728.
- ↑ Dongowski, G; Huth M, Gebhardt E, Flamme W (December 2002). "Dietary fiber-rich barley products beneficially affect the intestinal tract of rats". The Journal of nutrition (United States: American Society of Nutritional Sciences) 132 (12): 3704–3714. ISSN 0022-3166. PMID 12468611.
- ↑ Battilana, P; Ornstein K, Minehira K, Schwarz JM, Acheson K, Schneiter P, Burri J, Jéquier E, Tappy L (May 2001). "Mechanisms of action of beta-glucan in postprandial glucose metabolism in healthy men". European journal of clinical nutrition (England: Nature Publishing Group) 55 (5): 327–333. doi:10.1038/sj.ejcn.1601160. PMID 11378805.
- ↑ Tsukada, C; Yokoyama H, Miyaji C, Ishimoto Y, Kawamura H, Abo T (January 2003). "Immunopotentiation of intraepithelial lymphocytes in the intestine by oral administrations of beta-glucan". Cellular immunology (United States: Academic Press) 221 (1): 1–5. doi:10.1016/S0008-8749(03)00061-3. PMID 12742376.
- ↑ Frey, A.; Frey A, Giannasca KT, Weltzin R, Giannasca PJ, Reggio H, Lencer WI, Neutra MR (1996-09-01). "Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting". The Journal of experimental medicine (United States: Rockefeller University Press) 184 (3): 1045–1059. doi:10.1084/jem.184.3.1045. PMC 2192803. PMID 9064322.
- ↑ Tsukagoshi, S; Tsukagoshi S, Hashimoto Y, Fujii G, Kobayashi H, Nomoto K, Orita K (June 1984). "Krestin (PSK)". Cancer treatment reviews (England: Saunders) 11 (2): 131–155. doi:10.1016/0305-7372(84)90005-7. PMID 6238674.
- ↑ Obayashi T, Yoshida M, Mori T, et al. (1995). "Plasma (13)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes". Lancet 345 (8941): 17–20. doi:10.1016/S0140-6736(95)91152-9.
- ↑ Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. (2005). "Multicenter clinical evaluation of the (1→3)β-D-glucan assay as an aid to diagnosis of fungal infections in humans". Clin Infect Dis 41 (5): 654–659. doi:10.1086/432470. PMID 16080087.
- ↑ Odabasi Z, Mattiuzzi G, Estey E, et al. (2004). "Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome". Clin Infect Dis 39 (2): 199–205. doi:10.1086/421944. PMID 15307029.
- ↑ Mennink-Kersten MASH, Warris A, Verweij PE (2006). "1,3-β-D-Glucan in patients receiving intravenous amoxicillin–clavulanic acid". NEJM 354 (26): 2834–2835. doi:10.1056/NEJMc053340. PMID 16807428.
- ↑ Mennink-Kersten MASH, Ruegebrink D, Verweij PE (2008). "Pseudomonas aeruginosa as a cause of 1,3-β-D-glucan assay reactivity". Clin Infect Dis 46 (12): 1930–1931. doi:10.1086/588563. PMID 18540808.
References
- ARTHUR O. TZIANABOS Polysaccharide Immunomodulators as Therapeutic Agents, Harvard Medical School, Boston, USA - 2000
External links
- beta-Glucans at the US National Library of Medicine Medical Subject Headings (MeSH)
| |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||