Beta-Methylamino-L-alanine
| beta-Methylamino-L-alanine | |
|---|---|
 | |
| Other names 2-Amino-3-methylaminopropanoic acid[citation needed] | |
| Identifiers | |
| CAS number | 16676-91-8 |
| PubChem | 28558, 6999077 R, 105089 S |
| ChemSpider | 26564 |
| KEGG | C08291 |
| MeSH | alpha-amino-beta-methylaminopropionate |
| ChEBI | CHEBI:73169 |
| ChEMBL | CHEMBL11488 |
| Jmol-3D images | {{#if:CNCC(N)c(:[o]):[oH]CNCC(N)C(O)=O|Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C4H10N2O2 |
| Molar mass | 118.13 g mol−1 |
| log P | −0.1 |
| Acidity (pKa) | 1.883 |
| Basicity (pKb) | 12.114 |
| Related compounds | |
| Related alkanoic acids | |
| Related compounds | Dimethylacetamide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
β-Methylamino-L-alanine, or BMAA is a non-proteinogenic amino acid produced by cyanobacteria.
Structure & Properties
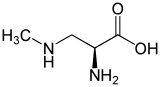
BMAA consists of an alpha carbon with a carboxyl and amino group with a methyl amino side chain. This non-proteinogenic amino acid is classified as a polar base.
Sources & Detection
BMAA is produced by cyanobacteria in marine, freshwater and terrestrial environments.[1][2] In cultured non-nitrogen-fixing cyanobacteria, BMAA production increases in nitrogen depleted medium.[3] BMAA has been found in aquatic organisms and in plants with cyanobacterial symbionts such as certain lichens, the floating fern Azolla, the leaf petioles of the tropical flowering plant Gunnera, cycads as well as in animals that eat the fleshy covering of cycad seeds, including flying foxes.[4][5][6][7]
A University of Miami study in the journal Marine Drugs discovered high concentrations of BMAA in shark fins. The study suggests that consumption of shark fin soup and cartilage pills may pose a health risk.[8]
The toxin can be detected via several laboratory methods, including liquid chromatography, high-performance liquid chromatography, mass spectrometry, amino acid analyzer, capillary electrophoresis and NMR spectroscopy.[9]
Neurotoxicity
Mechanisms
Although the mechanisms by which BMAA causes motor neuron dysfunction and death are not entirely understood, current research suggests that there are multiple mechanisms of action. Acutely, BMAA can act as an excitotoxin on glutamate receptors such as NMDA, calcium dependent AMPA and kainite receptors.[10][11] The activation of the metabotropic glutamate receptor 5 is believed to induce oxidative stress in the neuron by depletion of glutathione.[12]
BMAA is also known to misincorporate into nascent proteins in place of L-Serine, causing protein misfolding and aggregation, both hallmarks of tangle diseases, including Alzheimer's, Parkinson's and ALS, PSP and Lewy Body Disease. In-vitro research has shown that tRNA mischarging of L-BMAA for L-Serine can be inhibited in presence of excess L-Serine.[13]
Effects
Degenerative loco-motor diseases have been described in animals grazing on cycad species, fueling interest in a possible link between the plant and the etiology of ALS/PDC. Subsequent laboratory investigations discovered the presence of BMAA. BMAA induced severe neurotoxicity in rhesus macaques, including.[14]
- limb muscle atrophy
- nonreactive degeneration of anterior horn cells
- degeneration and partial loss of pyramidal neurons of the motor cortex
- behavioral dysfunction
- conduction deficits in the central motor pathway
- neuropathological changes of motor cortex Betz cells
There are reports that low BMAA concentrations can selectively kill cultured motor neurons from mouse spinal cords and produce reactive oxygen species.[11][15]
Human Cases
BMAA is considered a possible cause of the amyotrophic lateral sclerosis/parkinsonism–dementia complex (ALS/PDC) that had an extremely high rate of incidence among the Chamorro people of Guam.[16] The Chamorro call the condition lytico-bodig.[17] In the 1950s, ALS/PDC prevalence ratios and death rates for Chamorro residents of Guam and Rota were 50–100 times that of developed countries, including the United States.[17] No demonstrable heritable or viral factors were found for the disease, and a subsequent decline of ALS/PDC after 1963 on Guam, led to the search for responsible environmental agents.[18] The use of cycad (Cycas micronesica[19]) seeds in food decreased as the Chamorro population became more Americanized following World War II.[20]
In addition to eating the seeds directly, BMAA may be ingested by humans through biomagnification. Flying foxes, a Chamorro delicacy, may feed on cycad seeds and concentrate the toxin in their flesh. Twenty-four specimens of flying foxes from museum collections were tested for BMAA and BMAA was found in large concentrations in the flying foxes from Guam.[21]
Studies on human brain tissue of ALS/PDC, ALS, Alzheimer’s Disease, Parkinson’s Disease, Huntington’s Disease and neurological controls indicated that BMAA is present in non-genetic progressive neurodegenerative disease but not in controls or genetic-based Huntington’s Disease.[22][23][24][25]
There is currently ongoing research into the role of BMAA as an environmental factor in neurodegenerative disease.[26][27]
Therapeutic Target
Blocking the effects of BMAA in the brain and removing the toxin from the body are the goals of two United States Food and Drug Administration clinical drug trials aimed at treating ALS.[28][29]
See also
- Oxalyldiaminopropionic acid, a related toxin
References
- ↑ Cox, PA, Banack, SA, Murch, SJ, Rasmussen, U, Tien, G, Bidigare, RR, Metcalf, JS, Morrison, LF, Codd, GA, Bergman, B. (2005). "Diverse taxa of cyanobacteria produce b-N-methylamino-L-alanine, a neurotoxic amino acid". PNAS 102 (14). doi:10.1073/pnas.0501526102.
- ↑ Esterhuizen, M, Downing, TG. (2008). "β-N-methylamino-L-alanine (BMAA) in novel South African cyanobacterial isolates". Ecotoxicology and Environmental Safety 71 (2). doi:10.1016/j.ecoenv.2008.04.010.
- ↑ Downing, S, Banack, SA, Metcalf, JS, Cox, PA, Downing, TG. (2011). "Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-L-alanine". Toxicon 58: 187–194. doi:10.1016/j.toxicon.2011.05.017.
- ↑ Vega, A, Bell, A. (1967). "a-amino-β-methylaminopropionic acid, a new amino acid from seeds of cycas circinalis". Phytochemistry 6: 759–762.
- ↑ Banack, SA, Cox, PA. (2003). "Biomagnification of cycad neurotoxins in flying foxes: implications for ALS-PDC in Guam". Neurology 61 (3): 387–9.
- ↑ Masseret, E, Banack, S, Boumédiène, F, Abadie, E, Brient, L, Pernet, F, Juntas-Morales, R, Pageot, N, Metcalf, J, Cox, P, Camu, W. (2013). "Dietary BMAA exposure in an amyotrophic lateral sclerosis cluster from Southern France". PLOS ONE 8 (12). doi:10.1371/journal.pone.0083406.
- ↑ Field, NC, Metcalf, JS, Caller, TA, Banack, SA, Cox, PA, Stommel, EW. (2013). "Linking β-methylamino-L-alanine exposure to sporadic amyotrophic lateral sclerosis in Annapolis, MD". Toxicon 70: 179–183. doi:10.1016/j.toxicon.2013.04.010.
- ↑ http://www.sciencedaily.com/releases/2012/02/120223182516.htm=
- ↑ Cohen, SA. (2012). "Analytical techniques for the detection of a-amino- β-methylaminopropionic acid". Analyst 137. doi:10.1039/c2an16250d.
- ↑ Weiss, JH, Koh, J, Choi. D. (1989). "Neurotoxicity of β -N-methylamino-L-alanine (BMAA) and β-N-oxalylamino-L-alanine (BOAA) on cultured cortical neurons". Brain Research 497.
- ↑ 11.0 11.1 Lobner, D, Piana, PM, Salous, AK, Peoples, RW. (2007). "β-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms". Neurobiology of Disease 25 (2). doi:10.1016/j.nbd.2006.10.002.
- ↑ Rush, T, Liu, X, Lobner, D. (2012). "Synergistic toxicity of the environmental neurotoxins methylmercury and β-N-methylamino-L-alanine". Neuropharmacology and neurotoxicology 23. doi:10.1097/WNR.0b013e32834fe6d6.
- ↑ Dunlop, R.A., Cox, P.A., Banack, S.A., Rodgers, J.K. (2013). "The Non-Protein Amino Acid BMAA Is Misincorporated into Human Proteins in Place of l-Serine Causing Protein Misfolding and Aggregation". PLOS One 8 (9). doi:10.1371/journal.pone.0075376.
- ↑ Spencer, PS, Hugon, J, Ludolph, A, Nunn, PB, Ross, SM, Roy, DN, Schaumburg, HH. (1987). "Discovery and partial characterization of primate motor-system toxins". Ciba foundation symposium.
- ↑ Rao, SD, Banack, SA, Cox, PA, Weiss, JH. (2006). "BMAA selectively injures motor neurons via AMPA/kainate receptor activations". Experimental Neurology 201 (1). doi:10.1016/j.expneurol.2006.04.017.
- ↑ Cox, PA, Sacks, OW. (2002). "Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam". Neurology 58: 956–9.
- ↑ 17.0 17.1 Kurland, LK, Mulder, DW. (1954). "Epidemiologic investigations of amyotrophic lateral sclerosis". Neurology 4.
- ↑ Galasko, D, Salmon, DP, Craig, UK, Thal, LJ, Schellenberg, G, Wiederholt, W. (2002). "Clinical features and changing patterns of neurodegenerative disorders on Guam, 1997-2000". Neurology 58: 90–7.
- ↑ Hill, KD. (1994). "The cycas rumphii complex (Cycadeceae) in New Guinea and the Western Pacific". Australian systematic botany 7: 543–567.
- ↑ Whiting, MG. (1963). "Toxicity of cycads". Economic Botany 17 (4): 270–302.
- ↑ Banack, SA, Murch, SJ, Cox, PA. (2006). "Neurotoxic flying foxes as dietary items for the Chamorro people, Mariana Islands". Ethnopharmacology 106: 97–104. doi:10.1016/j.jep.2005.12.032.
- ↑ Murch, SJ, Cox, PA, Banack, SA. (2004). "A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam". PNAS 101 (33): 12228–12231. doi:10.1073/pnas.0404926101.
- ↑ Murch, SJ, Cox, PA, Banack, SA, Steele, JC, Sacks, OW. (2004). "Occurrence of b-methylamino-L-alanine (BMAA) in ALS/PDC patients from Guam". Acta Neurologica Scandinavica. doi:10.1111/j.1600-0404.2004.00320.x.
- ↑ Pablo, J, Banack, SA, Cox, PA, Johnson, TE, Papapetropoulos, Bradley, WG, Buck, A, Mash, DC. (2009). "Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease". Acta neurologica scandinavica 120: 215–225. doi:10.1111/j.1600-0404.2008.01150.x.
- ↑ Bradley, WG, Mash, DC. (2009). "Beyond Guam: the cyanobacterial/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases". ALS 10: 7–20. doi:10.3109/17482960903286009.
- ↑ Banack, SA, Caller, TA, Stommel, EW. (2010). "The cyanobacteria derived toxin beta-n-methylamino-L-alanine and Amyotrophic Lateral Sclerosis". Toxins 2: 2837–2850. doi:10.3390/toxins2122837.
- ↑ Holtcamp, W. (2012). "The emerging science of BMAA: do cyanobacteria contribute to neurodegenerative disease?". Environmental health perspective 120 (3). doi:10.1289/ehp.120-a110.
- ↑ http://www.clinicaltrials.gov/ct2/show/NCT01835782?term=serine+als&rank=1
- ↑ http://www.clinicaltrials.gov/ct2/show/NCT01259050?term=zinc+als&rank=1