Bergman cyclization
The Bergman cyclization or Bergman reaction or Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen donor (Scheme 1).[1] It is the most famous and well-studied member of the general class of cycloaromatization reactions.[2] It is named for the American chemist Robert George Bergman (b. 1942). The reaction product is a derivative of benzene.
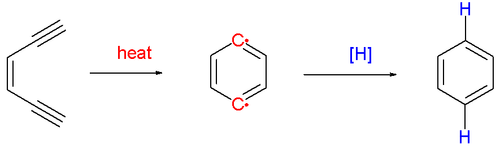
The reaction proceeds by a thermal reaction or pyrolysis (above 200°C) forming a short-lived and very reactive para-benzyne biradical species. It will react with any hydrogen donor such as 1,4-cyclohexadiene which converts to benzene. When quenched by tetrachloromethane the reaction product is a 1,4-dichlorobenzene and with methanol the reaction product is benzyl alcohol.
When the enyne moiety is incorporated into a 10-membered hydrocarbon ring (e.g. cyclodeca-3-ene-1,5-diyne in scheme 2) the reaction, taking advantage of increased ring strain in the reactant, is possible at the much lower temperature of 37°C.

Naturally occurring compounds such as calicheamicin contain the same 10-membered ring and are found to be cytotoxic. These compounds generate the diradical intermediate described above which can cause single and double stranded DNA cuts. There are novel drugs which attempt to make use of this property, including monoclonal antibodies such as mylotarg.[3]
A biradical mechanism is also proposed for the formation of certain biomolecules found in marine sporolides that have a chlorobenzene unit as part of their structure. In this mechanism a halide salt provides the halogen. A model reaction with the enediyene cyclodeca-1,5-diyn-3-ene, lithium bromide as halogen source and acetic acid as hydrogen source in DMSO at 37°C supports the theory:[4][5]

The reaction is found to be first-order in enediyne with the formation of p-benzyne A as the rate-limiting step. The halide ion then donates its two electrons in the formation of a new Br-C bond and radical electron involved is believed to shuttle over a transient C1-C4 bond forming the anion intermediate B. The anion is a powerful base, stripping protons even from DMSO to final product. The dibromide or dihydrogen product (tetralin) never form.
External links
- Bergman Cycloaromatization Powerpoint Whitney M. Erwin 2002
References
- ↑ p-Benzyne. Generation as an intermediate in a thermal isomerization reaction and trapping evidence for the 1,4-benzenediyl structure Richard R. Jones, Robert G. Bergman J. Am. Chem. Soc.; 1972; 94(2); 660-661. Abstract
- ↑ Mohamed, R. K.; Peterson, P. W.; Alabugin, I. V. Concerted Reactions that Produce Diradicals andZwitterions: Electronic, Steric, Conformational and Kinetic Control of Cycloaromatization Processes. Chem. Rev., 2013, 113 (9), pp 7089–7129, http://pubs.acs.org/doi/abs/10.1021/cr4000682
- ↑ Design and synthesis of heterocycle fused enediyne prodrugs activable at willLuca Banfi, Andrea Basso, Giuseppe Guanti, and Renata Riva Arkivoc 2006 HL-1786GR 261-275 Abstract
- ↑ Nucleophilic Addition to a p-Benzyne Derived from an Enediyne: A New Mechanism for Halide Incorporation into Biomolecules Charles L. Perrin, Betsy L. Rodgers, and Joseph M. O'Connor J. Am. Chem. Soc.; 2007; ASAP Web Release Date: 23-Mar-2007; (Article) doi:10.1021/ja070023e
- ↑ New Route For Halide Addition Stu Borman Chemical & Engineering News April 2 2007 Link