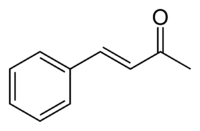
Benzylideneacetone
| Benzylideneacetone | |
|---|---|
 | |
 | |
| IUPAC name 4-Phenyl-3-buten-2-one | |
| Other names Benzalacetone | |
| Identifiers | |
| CAS number | 122-57-6 |
| ChemSpider | 21106584 |
| ChEBI | CHEBI:217301 |
| ChEMBL | CHEMBL73639 |
| RTECS number | EN0330000 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C10H10O |
| Molar mass | 146.19 g/mol |
| Appearance | pale yellow solid |
| Density | 1.008 g/cm3 |
| Melting point | 39–42 °C |
| Boiling point | 260–262 °C |
| Solubility in water | 1.3 g/L |
| Solubility in other solvents | nonpolar solvents |
| Hazards | |
| R-phrases | 36/37/38-43 |
| S-phrases | 22-26-36/37 |
| Main hazards | irritant |
| Flash point | 116 °C; 241 °F; 389 K |
| Related compounds | |
| Related compounds | Dibenzylideneacetone cinnamaldehyde |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Benzylideneacetone is the organic compound described by the formula C6H5CH=CHC(O)CH3. Although both cis- and trans-isomers are possible for the α,β-unsaturated ketone, only the trans isomer is observed. Its original preparation demonstrated the scope of condensation reactions to construct new, complex organic compounds.[1]
Preparation
Benzylideneacetone can be efficiently prepared by the NaOH-induced condensation of the readily available reagents acetone and benzaldehyde:[2]
- CH3C(O)CH3 + C6H5CHO → C6H5CH=CHC(O)CH3 + H2O

Reactions
As with most methyl ketones, benzylidineacetone is moderately acidic at the alpha position, and it can be readily deprotonated to form the corresponding enolate[3]

The compound undergoes the reactions expected for its collection of functional groups: e.g., the double bond adds bromine, the heterodiene adds electron-rich alkenes in Diels-Alder reactions to give dihydropyrans, the methyl group undergoes further condensation with benzaldehyde to give dibenzylideneacetone, and the carbonyl forms hydrazones. It reacts with Fe2(CO)9 to give (benzylideneacetone)Fe(CO)3, a reagent for transferring the Fe(CO)3 unit to other organic substrates.[4]
References
- ↑ Claisen, L. "Über die Einwirkung von Aceton auf Furfural und auf Benzaldehyd bei Gegenwart von Alkalilauge" Berichte der deutschen chemischen Gesellschaft 1881, volume 14, p 2468-2471.
- ↑ Drake, N. L.; Allen, Jr. P., "Benzalacetone", Org. Synth.; Coll. Vol. 1: 77
- ↑ Danheiser, R. L.; Miller, R. F.; Brisbois, R. G. (1990), "Detrifluoroacetylative Diazo Group Transfer: (E)-1-Diazo-4-phenyl-3-buten-2-one", Org. Synth. 73: 134; Coll. Vol. 9: 197
- ↑ Knölker, H.-J. "(η4-Benzylideneacetone)tricarbonyliron" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. Onlinedoi:10.1002/047084289X.rb058.