Benzilic acid rearrangement
The benzilic acid rearrangement is the rearrangement reaction of benzil with potassium hydroxide to benzilic acid. First performed by Justus Liebig in 1838 [1] this reaction type is displayed by 1,2-diketones in general. The reaction product is an α-hydroxy-carboxylic acid.[2]
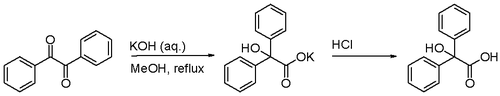
Certain acyloins also rearrange in this fashion.
This diketone reaction is related to other rearrangements: the corresponding keto-aldehyde (one alkyl group replaced by hydrogen) rearranges in a Cannizzaro reaction, the corresponding 1,2-diol reacts in a pinacol rearrangement.
Reaction mechanism
The reaction is a representative of 1,2-rearrangements. These rearrangements usually have migrating carbocations but this reaction is unusual because it involves a migrating carbanion. The long established reaction mechanism updated with in silico data [3] is outlined in scheme 2.
A hydroxide anion attacks one of the ketone groups in 1 in a nucleophilic addition to the hydroxyl anion 2. The next step requires a bond rotation to conformer 3 which places the migrating group R in position for attack on the second carbonyl group in a concerted step with reversion of the hydroxyl group back to the carbonyl group. This sequence resembles a nucleophilic acyl substitution. Calculations show that when R is methyl the charge build-up on this group in the transition state can be as high as 0.22 and that the methyl group is positioned between the central carbon carbon at a separation of 209 pm.
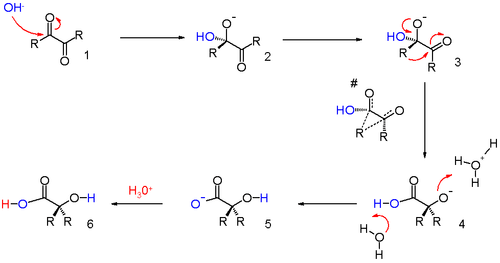
The carboxylic acid in intermediate 4 is less basic than the hydroxyl anion and therefore proton transfer takes place to intermediate 5 which can be protonated in acidic workup to the final α-hydroxy-carboxylic acid 6. Calculations show that an accurate description of the reaction sequence is possible with the participation of 4 water molecules taking responsibility for the stabilization of charge buildup. They also provide a shuttle for the efficient transfer of one proton in the formation of intermediate 5.
From a molecular orbital point of view this rearrangement may at a first glance not obvious. Contrary to a carbocationic rearrangement as in the Wagner-Meerwein rearrangement in which the empty carbocationic orbital interacts positively and symmetry allowed with the filled pi orbital HOMO of the central C-C bond (situation A in scheme 3), a filled carbanionic orbital should not be able to escape a symmetry forbidden MO overlap with the LUMO which is the empty antibonding pi orbital having one node (situation B).

In reality a 1,2-diketone LUMO is a 4 electron system without any nodes in the central C-C bond and a symmetry allowed transition is possible (Situation C). In other words the transition states of both a carbocationic rearrangement and the benzilic rearrangement obey the Woodward-Hoffmann rules because the involves respectively 2 electrons and 6 electrons (n=0 and 1 in the 4n+2 Hückel's rule).
Variations
A variation of this reaction occurs in certain steroids. In the so-called D-Homo Rearrangement of Steroids a cyclopentane ring expands to a cyclohexane ring with added base.[4][5]
References
- ↑ Liebig, J. (1838). "Ueber Laurent's Theorie der organischen Verbindungen". Annalen der Chemie 25: 1–31. doi:10.1002/jlac.18380250102.
- ↑ Donald A. Ballard and William M. Dehn (1941), "Benzilic acid", Org. Synth.; Coll. Vol. 1: 89
- ↑ Shinichi Yamabe, Noriko Tsuchida, and Shoko Yamazaki (2006). "A FMO-Controlled Reaction Path in the Benzil-Benzilic Acid Rearrangement". J. Org. Chem. 71 (5): 1777–1783. doi:10.1021/jo051862r. PMID 16496961.
- ↑ Über Steroide und Sexualhormone. 48. Mitteilung. Die Überführung von 17-Äthinyl-androsten-Derivaten in Pregnenon-Derivate. Herstellung des 17-Oxy-progesterons Helvetica Chimica Acta Volume 21, Issue 1, Date: 1938, Pages: 1760–1770 L. Ruzicka, H. F. Meldahl doi:10.1002/hlca.193802101214
- ↑ Über Steroide und Sexualhormone. (51. Mitteilung). Die Herstellung von Neo-pregnenolon aus 5-3, 17-Dioxypregnenon-(20) Helvetica Chimica Acta Volume 22, Issue 1, Date: 1939, Pages: 421–424 L. Ruzicka, H. F. Meldahl doi:10.1002/hlca.19390220155
