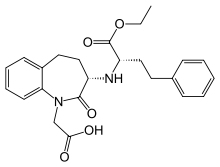
Benazepril
 | |
|---|---|
| Systematic (IUPAC) name | |
| 2-[(3S)-3-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}- | |
| Clinical data | |
| Trade names | Lotensin |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a692011 |
| Pregnancy cat. | D |
| Legal status | ℞ Prescription only |
| Routes | Oral |
| Pharmacokinetic data | |
| Protein binding | 96.7% |
| Metabolism | Hepatic glucuronidation |
| Half-life | 10-11 hours |
| Excretion | Renal and biliary |
| Identifiers | |
| CAS number | 86541-75-5 |
| ATC code | C09AA07 |
| PubChem | CID 5362124 |
| DrugBank | DB00542 |
| ChemSpider | 4514935 |
| UNII | UDM7Q7QWP8 |
| ChEBI | CHEBI:3011 |
| ChEMBL | CHEMBL838 |
| Chemical data | |
| Formula | C24H28N2O5 |
| Mol. mass | 424.49 g/mol |
| SMILES
| |
| |
| | |
Benazepril, brand name Lotensin (Novartis), is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure. Upon cleavage of its ester group by the liver, benazepril is converted into its active form benazeprilat, a non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor.
Dosage forms
Benazepril is available as oral tablets, in 5-, 10-, 20-, and 40-mg doses.
Benazepril is also available in combination with hydrochlorothiazide, under the trade name Lotensin HCT, and with amlodipine (trade name Lotrel).
Side effects
Most commonly, headaches and cough can occur with its use. Anaphylaxis, angioedema and hyperkalemia, the elevation of potassium levels, can also occur.
Benazepril may cause harm to the fetus during pregnancy.
Contraindications
Benazepril should be discontinued during pregnancy.
Kidney disease
According to a 2006 article in the New England Journal of Medicine, patients with advanced renal insufficiency taking benazepril showed "substantial" kidney benefits.[1]
A long-term study of patients' kidney disease revealed patients who took benazepril had better kidney function and slower progressions of kidney disease than their peers who took a placebo drug.[2] This is notable because this category of pharmaceuticals has long been thought to cause further kidney damage or increase the rate of progression for kidney disease.
According to coverage of the study on WebMD:
| “ | ACE inhibitors can pose a potential threat to kidneys as well. The key question was whether damaged kidneys would worsen if patients took ACE inhibitors. In a nutshell, concerns centered on blood levels of potassium and creatinine, waste products that are excreted by the kidneys. Testing creatinine levels in the blood is used as a way to monitor kidney function (...) kidney problems worsened more slowly in those taking Lotensin. Overall, there were no major differences in side effects between patients taking Lotensin or the placebo.[2] | ” |
This study marks the first indication that benazepril, and perhaps other ACE inhibitors, may actually be beneficial in the treatment of hypertension in patients with kidney disease.
Veterinary use
Under the brand names Fortekor (Novartis) and VetACE (Jurox Animal Health),[citation needed] benazepril hydrochloride is used to treat congestive heart failure in dogs[3][4] and chronic renal failure in dogs and cats.[citation needed]
References
- ↑ Hou F, Zhang X, Zhang G, Xie D, Chen P, Zhang W, Jiang J, Liang M, Wang G, Liu Z, Geng R (2006). "Efficacy and safety of benazepril for advanced chronic renal insufficiency". N Engl J Med 354 (2): 131–40. doi:10.1056/NEJMoa053107. PMID 16407508.
- ↑ 2.0 2.1 Hitti, Miranda; Chang, Louise (January 11, 2006). "Drug May Treat Advanced Kidney Disease". WebMD. Retrieved 2006-09-07.
- ↑ King JN, Mauron C, Kaiser G (December 1995). "Pharmacokinetics of the active metabolite of benazepril, benazeprilat, and inhibition of plasma angiotensin-converting enzyme activity after single and repeated administrations to dogs". Am. J. Vet. Res. 56 (12): 1620–8. PMID 8599524.
- ↑ O'Grady MR, O'Sullivan ML, Minors SL, Horne R (2009). "Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman Pinschers". J. Vet. Intern. Med. 23 (5): 977–83. doi:10.1111/j.1939-1676.2009.0346.x. PMID 19572914.
External links
| ||||||||||||||||||||