Bathochromic shift
Bathochromic shift is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength (lower frequency).[1]
Because the red color in the visible spectrum has a longer wavelength than most other colors, this effect is also commonly called a red shift, although this usage is considered informal,[2] and has no relation to Doppler shift or other wavelength-independent meanings of redshift. This usage is often found in the scientific literature.
This can occur because of a change in environmental conditions: for example, a change in solvent polarity will result in solvatochromism. A series of structurally related molecules in a substitution series can also show a bathochromic shift. Bathochromic shift is a phenomenon seen in molecular spectra, not atomic spectra; it is thus more common to speak of the movement of the peaks in the spectrum rather than lines.
 where
where 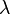 is the wavelength of the spectral peak of interest and
is the wavelength of the spectral peak of interest and 
Bathochromic shift is typically demonstrated using a spectrophotometer, colorimeter, or spectroradiometer.
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "bathochromic shift (effect)".
- ↑ Glossary of Terms Used in Photochemistry
See also
- Hypsochromic shift, a change to shorter wavelength (higher frequency)