Basic beryllium acetate
| Basic beryllium acetate | ||
|---|---|---|
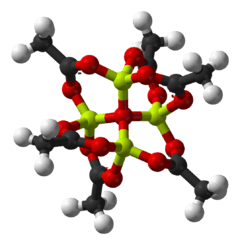 | ||
| IUPAC name Hexa-μ-acetato(O,O')-μ4-oxo teraberyllium(II)[1] | ||
| Identifiers | ||
| CAS number | 543-81-7 | |
| Properties | ||
| Molecular formula | C12H18Be4O13 | |
| Appearance | colorless | |
| Melting point | 285 °C | |
| Boiling point | 330 °C | |
| Solubility in chloroform | soluble | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Basic beryllium acetate is the chemical compound with the formula Be4O(O2CCH3)6. Although this compound has no applications and has been only lightly studied, it adopts a distinctive structure. "Basic acetates" consist of an ensemble of metal atoms, a central oxide atom, and an exterior of acetate groups. Another family of basic acetates are trimetallic with the formula M3O(O2CCH3)6(H2O)3 (M = Cr, Fe, Ru). Mixed metal members of this family also exist.
Preparation
To prepare Be4O(O2CCH3)6, basic beryllium carbonate is treated with hot acetic acid. The basic beryllium acetate is insoluble in water but soluble in chloroform, consistent with it being nonpolar. It melts and sublimes in a vacuum without decomposition.[2]
Structure
The structure of Be4O(O2CCH3)6 is relevant to its considerable stability. It is diamondoid, consisting of interlocking six-membered Be2O3C rings. The structure of this compound has been examined by two famous crystallography laboratories.[3][4]
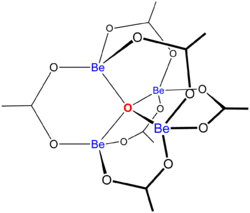
References
- ↑ Lee, J. D. (2008). Concise Inorganic Chemistry (5th ed.). Wiley India. p. 232. ISBN 978-81-265-1554-7.
- ↑ Moeller, T. (1950). "Basic Beryllium Derivatives of Organic Acids". In Audrieth, L. F. Inorganic Syntheses, Volume 3. John Wiley & Sons. p. 4. doi:10.1002/9780470132340.ch2. ISBN 978-0-470-13234-0.
- ↑ Bragg, W. H. (1923). "Crystal Structure of Basic Beryllium Acetate". Nature 111 (2790): 532. Bibcode:1923Natur.111..532B. doi:10.1038/111532a0.
- ↑ Pauling, L.; Sherman, J. (1934). "The Structure of the Carboxyl Group. II. The Crystal Structure of Basic Beryllium Acetate". Proceedings of the National Academy of Sciences 20 (6): 340. Bibcode:1934PNAS...20..340P. doi:10.1073/pnas.20.6.340.