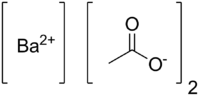
Barium acetate
| Barium acetate[1] | |
|---|---|
 | |
| IUPAC name Barium acetate | |
| Other names Barium diacetate | |
| Identifiers | |
| Abbreviations | Ba(OAc)2 |
| CAS number | 543-80-6 |
| ChemSpider | 10515 |
| UNII | FBA31YJ60R |
| Jmol-3D images | {{#if:[Ba+2].[O-]C(=O)C.[O-]C(=O)C|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C4H6BaO4 |
| Molar mass | 255.42 g mol−1 |
| Appearance | White solid |
| Odor | odorless |
| Density | 2.47 g/cm3 (anhydrous) 2.19 g/cm3/ (monohydrate) |
| Melting point | 450 °C; 842 °F; 723 K |
| Solubility in water | 55.8 g/100 mL (0 °C) 72 g/100mL (20 °C) |
| Solubility | slightly soluble in ethanol |
| Hazards | |
| Main hazards | Hazardous on ingestion |
| LD50 | 921 mg/kg (oral, rat) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Barium acetate (Ba(C2H3O2)2) is the salt of barium(II) and acetic acid.
Preparation
Barium acetate is generally produced by the reaction of acetic acid with barium carbonate:[2]
- BaCO3 + 2 CH3COOH → (CH3COO)2Ba + CO2 + H2O
The reaction is performed in solution and the barium acetate crystallizes out. Alternatively, barium sulfide can be used:[2]
Again, the solvent is evaporated off and the barium acetate crystallized.
Properties
Barium acetate is a white powder, which is highly soluble: at 0 °C, 55.8 g of barium acetate can be dissolved in 100 g of water. It decomposes upon heating into barium carbonate.[citation needed]
Reactions
When heated in air, barium acetate decomposes to the carbonate. It reacts with acids: reaction with sulfuric acid, hydrochloric acid and nitric acid give the sulfate, chloride and nitrate respectively.
Uses
Barium acetate is used as a mordant for printing textile fabrics, for drying paints and varnishes and in lubricating oil. In chemistry, it is used in the preparation of other acetates; and as a catalyst in organic synthesis.
References
- ↑ , JT Baker
- ↑ 2.0 2.1 Barium acetate, hillakomem.com, retrieved 30 June 2009
Further reading
- I. Gautier-Luneau; A. Mosset (1988). "Crystal structure of anhydrous barium acetate". Journal of Solid State Chemistry 73 (2): 473–479. Bibcode:1988JSSCh..73..473G. doi:10.1016/0022-4596(88)90133-8.
| |||||