Barbigerone
| Barbigerone | |
|---|---|
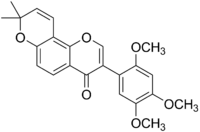 | |
| IUPAC name 3-(2,4,5-Trimethoxyphenyl)-8,8-dimethyl-4H,8H-benzo[1,2-b :3,4-b ']dipyran-4-one | |
| Other names Barubigeron; 2′,4′,5′-trimethoxy-6″,6″-dimethylpyrano(2″,3″:7,8)isoflavone | |
| Identifiers | |
| CAS number | 75425-27-3 |
| PubChem | 156793 |
| ChemSpider | 138031 |
| MeSH | C543999 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C23H22O6 |
| Molar mass | 394.42 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Barbigerone is one of a few pyranoisoflavones among several groups of isoflavones. It was first isolated from the seed of a leguminous plant Tephrosia barbigera; hence the name "barbigerone".[1] Members of the genus Millettia are now known to be rich in barbigerone, including M. dielsiena,[2] M. ferruginea,[3] M. usaramensis,[4] and M. pachycarpa.[5] It has also been isolated from the medicinal plant Sarcolobus globosus.[6] Barbigerone from S. globosus is validated to have significant antioxidant property.[7] Barbigerone exhibits profound antiplasmodial activity against the malarial parasite Plasmodium falciparum.[8] It is also demonstrated that it has anti-cancer potential as it causes apoptosis of murine lung-cancer cells.[9]
References
- ↑ Vilain C (1980). "Barbigerone, next term a new pyranoisoflavone from seeds of Tephrosia barbigera". Phytochemistry 19 (5): 988. doi:10.1016/0031-9422(80)85162-4.
- ↑ Gong, Ting; Wang, Dong-Xiao; Chen, Ruo-Yun; Liu, Ping; Yu, De-Quan (2009). "Novel benzil and isoflavone derivatives from Millettia dielsiana". Planta Medica 75 (3): 236–242. doi:10.1055/s-0028-1112203. PMID 19140097.
- ↑ Dagne E, Bekele A (1990). "C-prenylated isoflavones from Millettia ferruginea". Phytochemistry 29 (8): 2679–2682. doi:10.1016/0031-9422(90)85212-X.
- ↑ Yenesew, Abiy; Midiwo, Jacob O.; Waterman, Peter G. (1998). "Rotenoids, isoflavones and chalcones from the stem bark of Millettia usaramensis subspecies usaramensis". Phytochemistry 47 (2): 295. doi:10.1016/S0031-9422(97)00424-X.
- ↑ Ye, Haoyu; Zhong, Shijie; Li, Yanfang; Tang, Minghai; Peng, Aihua; Hu, Jia; Shi, Jie; He, Shicao; Wu, Wenshuang; Chen, Lijuan (2010). "Enrichment and isolation of barbigerone from Millettia pachycarpa Benth. using high-speed counter-current chromatography and preparative HPLC". Journal of Separation Science 33 (8): 1010–7. doi:10.1002/jssc.200900641. PMID 20187026.
- ↑ Wangensteen, H; Alamgir, M; Rajia, S; Samuelsen, AB; Malterud, KE (2005). "Rotenoids and isoflavones from Sarcolobus globosus". Planta Medica 71 (8): 754–758. doi:10.1055/s-2005-864182. PMID 16142641.
- ↑ Wangensteen, H; Miron, A; Alamgir, M; Rajia, S; Samuelsen, AB; Malterud, KE (2006). "Antioxidant and 15-lipoxygenase inhibitory activity of rotenoids, isoflavones and phenolic glycosides from Sarcolobus globosus". Fitoterapia 77 (4): 290–295. doi:10.1016/j.fitote.2006.03.0171. PMID 16701962.
- ↑ Yenesew, A; Derese, S; Midiwo, JO; Oketch-Rabah, HA; Lisgarten, J; Palmer, R; Heydenreich, M; Peter, MG; Akala, H; Wangui, J; Liyala, P; Waters, NC (2003). "Anti-plasmodial activities and X-ray crystal structures of rotenoids from Millettia usaramensis subspecies usaramensis". Phytochemistry 64 (3): 773–779. doi:10.1016/S0031-9422(03)00373-X. PMID 13679101.
- ↑ Li, ZG; Zhao, YL; Wu, X; Ye, HY; Peng, A; Cao, ZX; Mao, YQ; Zheng, YZ; Jiang, PD; Zhao, X; Chen, LJ; Wei, YQ (2009). "Barbigerone, a natural isoflavone, induces apoptosis in murine lung-cancer cells via the mitochondrial apoptotic pathway". Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 24 (1–2): 95–104. doi:10.1159/000227817. PMID 19590197.
External links
| |||||||||||||||||||||||