Barbier reaction
From Wikipedia, the free encyclopedia

Barbier reaction with samarium(II) iodide
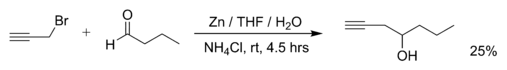
Examples of Barbier reactions are the reaction of propargylic bromide with butanal with zinc metal in water:[2]
the intramolecular Barbier reaction with samarium(II) iodide:[3]

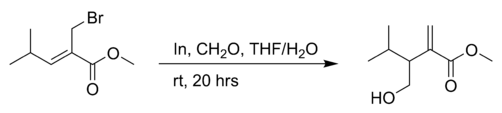
the reaction of an allyl bromide with formaldehyde in THF with indium powder:[4]
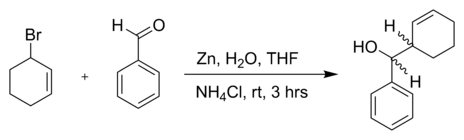
or another allyl bromide in a reaction with benzaldehyde and zinc powder in water:[5]
See also
- Grignard reaction
- Nozaki-Hiyama-Kishi reaction
- Indium mediated allylation
External links
- Barbier reaction @ University of Connecticut Website
References
- ↑ Barbier, P. (1899). "Synthèse du diéthylhepténol". Compt. Rend. 128: 110.
- ↑ Artur Jõgi and Uno Mäeorg (2001). "Zn Mediated Regioselective Barbier Reaction of Propargylic Bromides in THF/aq. NH4Cl Solution". Molecules 6 (12): 964–968. doi:10.3390/61200964. ISSN 1420-3049.
- ↑ Tore Skjæret and Tore Benneche (2001). "Preparation of oxo-substituted α-chloro ethers and their reaction with samarium diiodide". Arkivoc: KU–242A.
- ↑ George D. Bennett and Leo A. Paquette, "Methyl 3-(hydroxymethyl)-4-methyl-2-methylenepentanoate", Org. Synth.; Coll. Vol. 10: 77
- ↑ Gary W. Breton, John H. Shugart, Christine A. Hughey, Brian P. Conrad, Suzanne M. Perala (2001). "Use of Cyclic Allylic Bromides in the Zinc–Mediated Aqueous Barbier–Grignard Reaction". Molecules 6 (8): 655–662. doi:10.3390/60800655.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.