Baeyer–Villiger oxidation
The Baeyer–Villiger oxidation is an organic reaction in which a ketone is oxidized to an ester by treatment with peroxy acids or hydrogen peroxide.[1][2] Key features of the Baeyer–Villiger oxidation are its stereospecificity and predictable regiochemistry.[3] It is named after the German chemist Johann Friedrich Wilhelm Adolf von Baeyer (1835–1917) and the Swiss chemist Victor Villiger (1868–1934).[4] This reaction is also called Baeyer–Villiger rearrangement.

Reagents typically used to carry out this rearrangement are meta-chloroperoxybenzoic acid (mCPBA), peroxyacetic acid, or peroxytrifluoroacetic acid.[5] Reactive or strained ketones (cyclobutanones, norbornanones) react with hydrogen peroxide or hydroperoxides to form lactones. The original reagent in the 1899 publication is Caro's acid discovered just a year earlier.[6] Disodium phosphate or sodium bicarbonate is often added as a buffering agent to prevent transesterification or hydrolysis.
Mechanism
The reaction mechanism of this oxidative cleavage involves first addition of the peroxy acid to the carbonyl forming a tetrahedral intermediate also called the Criegee intermediate for its similarity with rearrangement of that name. The transition state for this step is envisioned as a hydrogen relay involving three peroxy acid molecules with linear O-H-O interactions.[7] Next is a concerted migration of one of the adjacent carbons to oxygen with loss of a carboxylic acid. If the migrating carbon is chiral, the stereochemistry is retained.
.png)
- Migratory aptitude: hydrogen > tertiary alkyl > secondary alkyl > phenyl > primary alkyl > methyl [8]
In the transition state for this migration step the R–C–O–O dihedral angle should be 180° to maximise the interaction between the filled R–C sigma bond and the antibonding O–O sigma bond. This step is also (at least in silico) assisted by two or three peroxyacid units enabling the hydroxyl proton to shuttle to its new position.[7]
For unsymmetrical ketones, the migrating group is usually the one that can best stabilize positive charge. Thus, cyclic ketones produce lactones and aldehydes usually produce carboxylic acids, although formates can also be formed if the migrating group is tertiary or an electron rich vinyl group or aromatic ring (Dakin reaction). Sometimes the alcohol is formed when the formate is hydrolytically unstable. A hydrogen –R group almost never migrates.
This mechanism was first proposed by Doering and Dorfman in 1953 and based on isotope labeling experiments[9][10]
Biocatalytic BV oxidation
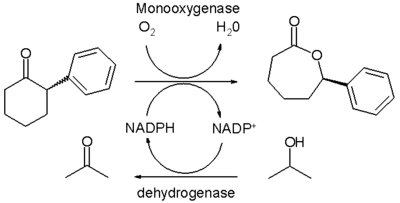
The Baeyer–Villiger oxidation can also be performed by biocatalysis with a so-called Baeyer-Villiger monooxygenase or BVMO. Though largely an experimental technique it shows the promise of enantioselectivity and green chemistry for this reaction type. Current stumbling blocks are confinement to water as the reaction medium, substrate specificity, dependence on the stoichiometric use and costs of cofactors such as NADPH and the costs associated with BVMO's themselves because lengthy purification steps are required. In vivo oxidations with metabolically active microbial cells introduce complications on their own.
In one study[11] the enzyme purification issue is addressed and a special thermally stable monooxygenase is isolated from a specific E. coli strain. This enzyme converts racemic 2-phenylcyclohexanone with oxygen to the corresponding (R)-lactone with 50% chemical yield and 94% enantiomeric excess within a biphasic system of water and hexane. The NADPH cofactor is regenerated in each catalytic cycle by action of a second dehydrogenase enzyme which consumes isopropanol as a sacrificial catalyst. The solubility of the organic reactant and product is low in the aqueous phase thus averting inhibition. On the other hand the catalytic turnover number for this reaction is much larger than can be obtained with classical organic asymmetric catalysts.
See also
- Dakin reaction
References
- ↑ Baeyer, A.; Villiger, V. (1899). "Einwirkung des Caro'schen Reagens auf Ketone" (abstract). Ber. 32 (3): 3625–33. doi:10.1002/cber.189903203151.
- ↑ Baeyer, A.; Villiger, V. (1900). "Ueber die Einwirkung des Caro'schen Reagens auf Ketone" (abstract). Ber. 33 (1): 858–64. doi:10.1002/cber.190003301153.
- ↑ Crudden, C. M.; Chen, A. C.; Calhoun, L. A. (2000). "A Demonstration of the Primary Stereoelectronic Effect in the Baeyer-Villiger Oxidation of α-Fluorocyclohexanones". Angew. Chem. Int. Ed. 39 (16): 2851–55. doi:10.1002/1521-3773(20000818)39:16<2851::AID-ANIE2851>3.0.CO;2-Y.
- ↑ Krow, G. R. Org. React. 1993, 43, 251. doi:10.1002/0471264180.or043.03
- ↑ Burton, J.W.; Clark, J.S.; Derrer, S.; Stork, T.C.; Bendall, J.G.; Holmes, A.B. (1997). "Synthesis of Medium Ring Ethers. 5. The Synthesis of (+)-Laurencin" (Abstract). J. Am. Chem. Soc. 119 (32): 7483–7498. doi:10.1021/ja9709132.
- ↑ Michael Renz, Bernard Meunier (1999). "100 Years of Baeyer-Villiger Oxidations". European Journal of Organic Chemistry 1999 (4): 737–50. doi:10.1002/(SICI)1099-0690(199904)1999:4<737::AID-EJOC737>3.0.CO;2-B.
- ↑ 7.0 7.1 The Role of Hydrogen Bonds in Baeyer-Villiger Reactions Shinichi Yamabe and Shoko Yamazaki J. Org. Chem.; 2007; 72(8) pp 3031–41; (Article) doi:10.1021/jo0626562
- ↑ http://classes.yale.edu/02-03/chem125a/125webSpring/BaeyerVilliger/migratoryaptitude.htm
- ↑ Mechanism of the Peracid Ketone—Ester Conversion. Analysis of Organic Compounds for Oxygen-181 W. von E. Doering, Edwin Dorfman J. Am. Chem. Soc., 1953, 75 (22), pp 5595–5598 doi:10.1021/ja01118a035
- ↑ 100 Years of Baeyer–Villiger Oxidations European Journal of Organic Chemistry Volume 1999, Issue 4, April 1999, Pages: 737–750, Michael Renz and Bernard Meunier doi: 10.1002/(SICI)1099-0690(199904)1999:4<737::AID-EJOC737>3.0.CO;2-B
- ↑ Frank Schulz; François Leca; Frank Hollmann; Manfred T Reetz (2005). "Towards practical biocatalytic Baeyer-Villiger reactions: applying a thermostable enzyme in the gram-scale synthesis of optically active lactones in a two-liquid-phase system" (PDF). Beilstein Journal of Organic Chemistry 1 (10): 10. doi:10.1186/1860-5397-1-10. PMC 1399458. PMID 16542025.