Dominance (genetics)
Dominance in genetics is a relationship between alleles of a single gene, in which one allele masks the phenotypic expression of a second allele at the same gene locus.[1] The first allele is said to be dominant and the second allele is said to be recessive. When the relevant gene resides on an ordinary chromosome (as opposed to a sex chromosome) the alleles and their associated traits may be referred to as autosomal dominant and autosomal recessive. Dominance is a key concept in Mendelian inheritance, which underlies classical genetics.
A classic example of dominance is the inheritance of seed shape (pea shape) in peas. Peas may be round (associated with an allele designated R) or wrinkled (associated with an allele designated W). In this case, three combinations of alleles (genotypes) are possible: RR, RW, and WW. The RR individuals have round peas and the WW individuals have wrinkled peas. In RW individuals the R allele masks the presence of the W allele, so these individuals also have round peas. Allele R is said to dominate allele W, and allele W is said to be recessive to allele R.
More generally, where a gene exists in two allelic versions (designated A and B), three combinations of alleles (genotypes) are possible: AA, AB, and BB. If AA and BB individuals (homozygotes) show different forms of some trait (phenotypes), and AB individuals (heterozygotes) show the same phenotype as AA individuals, then allele A is said to dominate or be dominant to or show dominance to allele B, and B is said to be recessive to A. If instead AB has the same phenotype as BB, B is said to be dominant to A.
Dominance is not inherent to an allele, it is a relationship between alleles; one allele can be dominant to a second allele, recessive to a third allele, and codominant to a fourth. Dominance should be distinguished from epistasis, a relationship in which an allele of one gene affects the expression of an allele at a different gene.
Background
Diploid / haploid
Most familiar animals and some plants have paired chromosomes, and are described as diploid. They have two versions of each chromosome, one contributed by the female parent in her ovum, and one by the male parent in his sperm, which are joined at fertilization. The ovum and sperm cells (the gametes) have only one copy of each chromosome and are described as haploid. Production of haploid gametes occurs through a process called meiosis.
Chromosomes, genes, and alleles


Each chromosome of a matching (homologous) pair is structurally similar to the other, and each member of a homologous pair has a very similar DNA sequence (loci, singular locus). The DNA in each chromosome functions as a series of discrete genes that influence various traits. Thus, each gene also has a corresponding homologue, which may exist in different versions called alleles. The alleles at the same locus on the two homologous chromosomes may be identical or different.
In popular use, "gene" and "allele" are often used interchangeably. This produces misunderstandings. Properly, "gene" refers to a hereditary unit, ordinarily at a fixed position on a chromosome, that influences a particular trait. Genes are sequences of DNA. "Allele" refers to any of the many particular versions of a gene that may be present in a population of individuals from a particular species, at a particular locus. E.g., it is inaccurate to say "This pea plant has a pair of wrinkled genes", and it is more accurate to say, "This plant has two 'w' alleles for the gene that influences seed shape, and will therefore produce wrinkled peas." Consider also the example of blood type in humans. Near the long arm of chromosome nine appears a gene that determines whether an individual will be blood type, A, B, or O. There are three different alleles that could be present at this locus, but only two can be present in any individual, one inherited from their mother and one from their father.[2]
Homozygous, heterozygous
If two alleles of a given gene are identical, the organism is called a homozygote and is said to be homozygous with respect to that gene; if instead the two alleles are different, the organism is a heterozygote and is heterozygous. The genetic makeup of an organism, either at a single locus or over all its genes collectively, is called its genotype. The genotype of an organism directly and indirectly affects its molecular, physical, and other traits, which individually or collectively are called its phenotype. At heterozygous gene loci, the two alleles interact to produce the phenotype. One important kind of allele interaction is that described by Mendel, now called Mendelian, in which the heterozygous individual has the appearance/phenotype caused by one allele when homozygous, called dominant, and not the appearance/phenotype caused by the other allele. The alleles in this case are said to be dominant and recessive to each other, respectively.
In complete dominance, the phenotypic effect of one allele completely masks the other in heterozygous combination; that is, the phenotype produced by the two alleles in heterozygous combination is identical to that produced by one of the two homozygous genotypes. The allele that masks the other is said to be dominant to the latter, and the alternative allele is said to be recessive to the former.[3]
For more information see Zygosity.
Which trait is dominant?
By definition, the terms dominant and recessive refer to the genotypic interaction of alleles in producing the phenotype of the heterozygote. The key concept is genetic: Which of the two alleles present in the heterozygote is expressed, such that the organism is phenotypically identical to one of the two homozygotes. It is sometimes convenient to talk about the trait corresponding to the dominant allele as the dominant trait, and the trait corresponding to the hidden allele as the recessive trait. However, this can easily lead to confusion in understanding the concept as phenotypic. For example, to say that "green peas" dominate "yellow peas" confuses inherited genotypes and expressed phenotypes, and will subsequently confuse discussion of the molecular basis of the phenotypic difference.
Dominance is not inherent. One allele can be dominant to a second allele, recessive to a third allele, and codominant to a fourth.
Dominance is unrelated to the nature of the phenotype itself, that is, whether it is regarded as "normal" or "abnormal," "standard" or "nonstandard," "healthy" or "diseased," "stronger" or "weaker," or more or less extreme. A dominant allele may account for any of these trait types. Other distinctions are made between the gene locus (e.g. "A gene influencing seed shape"), the alleles at that locus (e.g. the "round" or "wrinkled alleles"), and the phenotype the alleles produce (e.g. "round" or "wrinkled").
Nomenclature
In genetics, the common convention for pairs of alleles where one is dominant to the other is that dominant alleles are written as capital letters and recessive alleles as lower-case letters. In the pea example, once the dominance relationships of the two alleles are known, it is possible to designate the dominant allele that produces a round shape by a capital-letter symbol R, and the alternative recessive allele that produces a wrinkled shape by a lower-case symbol r. The homozygous dominant, heterozygous, and homozygous recessive genotypes are then written RR, Rr, and rr, respectively. It would also be possible to designate the two alleles as W and w, and the three genotypes WW, Ww, and ww, the first two of which produced round peas and the third wrinkled peas. Note that the choice of "R" or "W" as the symbol for the dominant allele does not pre-judge whether the allele causing the "round" or "wrinkled" phenotype when homozygous is the dominant one.
Relationship to other genetic concepts
The concept of dominance is involved with a number of other genetic concepts.
Multiple alleles
Although any individual of a diploid organism has at most two different alleles at any one locus, most genes exist in a large number of allelic versions in the population as a whole. If the alleles have different effects on the phenotype, sometimes their dominance interactions with each other can be described as a series.
For example, coat color in domestic cats is affected by a series of alleles of the TYR gene (which encodes the enzyme tyrosinase). The alleles C, cb, cs, and ca (full colour, burmese, siamese, and albino, respectively) produce different levels of pigment and hence different levels of color dilution. The C allele (full colour) is completely dominant to the last three and the ca allele (albino) is completely recessive to the first three. [4] [5] [6]
Complete dominance
Complete dominance occurs when the phenotype of the heterozygote is completely indistinguishable from that of the dominant homozygote.
Incomplete and semi-dominance
Incomplete dominance occurs when the phenotype of the heterozygous genotype is distinct from and often intermediate to the phenotypes of the homozygous genotypes. For example, the snapdragon flower color is either homozygous for red or white. When the red homozygous flower is paired with the white homozygous flower, the result yields a pink snapdragon flower. The pink snapdragon is the result of incomplete dominance. A similar type of incomplete dominance is found in the four o'clock plant wherein pink color is produced when true-bred parents of white and red flowers are crossed. In quantitative genetics, where phenotypes are measured and treated numerically, if a heterozygote's phenotype is exactly between (numerically) that of the two homozygotes, the phenotype is said to exhibit no dominance at all, i.e. dominance exists only when the heterozygote's phenotype measure lies closer to one homozygote than the other.
When plants of the F1 generation are self-pollinated, the phenotypic and genotypic ratio of the F2 generation will be 1:2:1 (Red:Pink:White) for both generations.[7]
Co-dominance


Co-dominance occurs when the contributions of both alleles are visible in the phenotype. For example, in the ABO blood group system, chemical modifications on the surfaces of blood cells are controlled by three alleles (IA, IB and IO) at the ABO locus. The IA and IB alleles produce different modifications, and the non-functional IO allele produces no modification. Thus IA and IB alleles are each dominant to IO (IA IA and IA IO individuals both have type A blood, and IB IB and IB IO individuals both have type B blood. But IA IB individuals have both modifications on their blood cells and thus have type AB blood, so the IA and IB alleles are said to be co-dominant.)
Another example occurs at the locus for the Beta-globin component of hemoglobin, where the three molecular phenotypes of HbA/HbA, HbA/HbS, and HbS/HbS are all distinguishable by protein electrophoresis. (The medical condition produced by the heterozygous genotype is called sickle-cell trait and is a milder condition distinguishable from sickle-cell anemia, thus the alleles show incomplete dominance with respect to anemia, see above). For most gene loci at the molecular level, both alleles are expressed co-dominantly, because both are transcribed into RNA.
Co-dominance, where allelic products co-exist in the phenotype, is different from incomplete or semi-dominance, where the quantitative interaction of allele products produces an intermediate phenotype. For example in Co-dominance, a red homozygous flower and a white homozygous flower will produce offspring that have red and white spots. When plants of the F1 generation are self-pollinated, the phenotypic and genotypic ratio of the F2 generation will be 1:2:1 (Red:Spotted:White). These ratios are the same as those for incomplete dominance. Again, note that this classical terminology is inappropriate - in reality such cases should not be said to exhibit dominance at all.
Autosomal versus sex-linked dominance
In humans and other mammal species, sex is determined by two sex chromosomes called the X chromosome and the Y chromosome. Human females are typically XX; males are typically XY. The remaining pairs of chromosome are found in both sexes and are called autosomes; genetic traits due to loci on these chromosomes are described as autosomal, and may be dominant or recessive. Genetic traits on the X and Y chromosomes are called sex linked, because they tend to be characteristic of one sex or the other. In practice, the term almost always refers to X-linked traits. Females have two copies of every gene locus found on the X chromosome, just as for the autosomes, and the same dominance relationships apply. Males however have only one copy of each X chromosome gene locus, and are described as hemizygous for these genes. The Y chromosome is much smaller than the X, and contains a much smaller set of genes that influence 'maleness', such as the SRY gene for testis determining factor. Dominance rules for sex-linked gene loci are determined by their behavior in the female: because the male has only one allele, that allele is always expressed regardless of whether it is dominant or recessive.
Epistasis
Epistasis ["epi + stasis = to sit on top"] is an interaction between alleles at two different gene loci that affect a single trait, which may sometimes resemble a dominance interaction between two different alleles at the same locus. Epistasis modifies the characteristic 9:3:3:1 ratio expected for two non-epistatic genes. For two loci, 14 classes of epistatic interactions are recognized. As an example of recessive epistasis, one gene locus may determine whether a flower pigment is yellow (AA or Aa) or green (aa), while another locus determines whether the pigment is produced (BB or Bb) or not (bb). In a bb plant, the flowers will be white, irrespective of the genotype of the other locus as AA, Aa, or aa. The bb combination is not dominant to the A allele: rather, the B gene shows recessive epistasis to the A gene, because the B locus when homozygous for the recessive allele (bb) suppresses phenotypic expression of the A locus. In a cross between two AaBb plants, this produces a characteristic 9:3:4 ratio, in this case of yellow : green : white flowers.
In dominant epistasis, one gene locus may determine yellow or green pigment as in the previous example: AA and Aa are yellow, and aa are green. A second locus determines whether a pigment precursor is produced (dd) or not (DD or Dd). Here, in a DD or Dd plant, the flowers will be colorless irrespective of the genotype at the A locus, because of the epistatic effect of the dominant D allele. Thus, in a cross between two AaDd plants, 3/4 of the plants will be colorless, and the yellow and green phenotypes are expressed only in dd plants. This produces a characteristic 12:3:1 ratio of white : yellow : green plants.
Supplementary epistasis occurs when two loci affect the same phenotype. For example, if pigment color is produced by CC or Cc but not cc, and by DD or Dd but not dd, then pigment is not produced in any genotypic combination with either cc or dd. That is, both loci must have at least one dominant allele to produce the phenotype. This produces a characteristic 9:7 ratio of pigmented to unpigmented plants. Complementary epistasis in contrast produces an unpigmented plant if and only if the genotype is cc and dd, and the characteristic ratio is 15:1 between pigmented and unpigmented plants.[8]
Classical genetics considered epistatic interactions between two genes at a time. It is now evident from molecular genetics that all gene loci are involved in complex interactions with many other genes (e.g., in metabolic pathways may involve scores of genes), and that this creates epistatic interactions that are much more complex than the classic two-locus models.
Hardy-Weinberg principle (Estimation of carrier frequency)
The frequency of the homzygous state (which is the carrier state for a recessive trait) can be estimated using the Hardy-Weinberg formula: 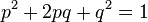
This formula applies to a gene with exactly two alleles and relates the frequencies of those alleles in a large population to the frequencies of their three genotypes in that population.
For example, if p is the frequency of allele A, and q is the frequency of allele a then the terms p2, 2pq, and q2 are the frequencies of the genotypes AA, Aa and aa respectively. Since the gene has only two alleles, all alleles must be either A or a and p + q = 1. Now, if A is completely dominant to a then the frequency of the carrier genotype Aa cannot be directly observed (since it has the same traits as the homozygous genotype AA), however it can be estimated from the frequency of the recessive trait in the population, since this is the same as that of the homozygous genotype aa. i.e. the individual allele frequencies can be estimated: q = √<var>f</var>(aa), p = 1 - q, and from those the frequency of the carrier genotype can be derived: <var>f</var>(Aa) = 2pq.
This formula relies on a number of assumptions and an accurate estimate of the frequency of the recessive trait. In general, any real-world situation will deviate from these assumptions to some degree, introducing corresponding inaccuracies into the estimate. If the recessive trait is rare, then it will be hard to estimate its frequency accurately, as a very large sample size will be needed.
Molecular mechanisms
The molecular basis of dominance was unknown to Mendel. It is now understood that a gene locus includes a long series (hundreds to thousands) of bases or nucleotides of deoxyribonucleic acid (DNA) at a particular point on a chromosome. The central dogma of molecular biology states that "DNA makes RNA makes protein", that is, that DNA is transcribed to make an RNA copy, and RNA is translated to make a protein. In this process, different alleles at a locus may or may not be transcribed, and if transcribed may be translated to slightly different versions of the same protein (called isoforms). Proteins often function as enzymes that catalyze chemical reactions in the cell, which directly or indirectly produce phenotypes. In any diploid organism, the DNA sequences of the two alleles present at any gene locus may be identical (homozygous) or different (heterozygous). Even if the gene locus is heterozygous at the level of the DNA sequence, the proteins made by each allele may be identical. In the absence of any difference between the protein products, neither allele can be said to be dominant (see co-dominance, above). Even if the two protein products are slightly different (allozymes), it is likely that they produce the same phenotype with respect to enzyme action, and again neither allele can be said to be dominant.
Dominance typically occurs when one of the two alleles is non-functional at the molecular level, that is, it is not transcribed or else does not produce a functional protein product. This can be the result of a mutation that alters the DNA sequence of the allele. An organism homozygous for the non-functional allele will generally show a distinctive phenotype, due to the absence of the protein product. For example, in humans and other organisms, the unpigmented skin of the albino phenotype[9] results when an individual is homozygous for an allele that prevents synthesis of the skin pigment protein melanin. It is important to understand that it is not the lack of function that allows the allele to be described as recessive: this is the interaction with the alternative allele in the heterozygote. Three general types of interaction are possible:
- In the typical case, the single functional allele makes sufficient protein to produce a phenotype identical to that of the homozygote: this is called haplosufficiency. For example, suppose the standard amount of enzyme produced in the functional homozygote is 100%, with the two functional alleles contributing 50% each. The single functional allele in the heterozygote produces 50% of the standard amount of enzyme, which is sufficient to produce the standard phenotype. If the heterozygote and the functional-allele homozygote have identical phenotypes, the functional allele is dominant to the non-functional allele. This occurs at the albino gene locus: the heterozygote produces sufficient enzyme to convert the pigment precursor to melanin, and the individual has standard pigmentation.
- Less commonly, the presence of a single functional allele gives a phenotype that is not normal but less severe than that of the non-functional homozygote. This occurs when the functional allele is not haplo-sufficient. The terms haplo-insufficiency and incomplete dominance are typically applied to these cases. The intermediate interaction occurs where the heterozygous genotype produces a phenotype intermediate between the two homozygotes. Depending on which of the two homozygotes the heterozygote most resembles, one allele is said to show incomplete dominance over the other. For example, in humans the Hb gene locus is responsible for the Beta-chain protein (HBB) that is one of the two globin proteins that make up the blood pigment hemoglobin[9] Many people are homozygous for an allele called HbA; some persons carry an alternative allele called HbS, either as homozygotes or heterozygotes. The hemoglobin molecules of HbS/HbS homozygotes undergo a change in shape that distorts the morphology of the red blood cells, and causes a severe, life-threatening form of anemia called sickle-cell anemia. Persons heterozygous HbA/HbS for this allele have a much less severe form of anemia called sickle-cell trait. Because the disease phenotype of HbA/HbS heterozygotes is more similar to but not identical to the HbA/HbA homozygote, the HbA allele is said to be incompletely dominant to the HbS allele.
- Rarely, a single functional allele in the heterozygote may produce insufficient gene product for any function of the gene, and the phenotype resembles that of the homozygote for the non-functional allele. This complete haploinsufficiency is very unusual. In these cases the non-functional allele would be said to be dominant to the functional allele. This situation may occur when the non-functional allele produces a defective protein that interferes with the proper function of the protein produced by the standard allele. The presence of the defective protein "dominates" the standard protein, and the disease phenotype of the heterozygote more closely resembles that of the homozygote for two defective alleles. Note that the term dominant is often incorrectly applied to defective alleles whose homozygous phenotype has not been examined, but which cause a distinct phenotype when heterozygous with the normal allele. This phenomenon occurs in a number of trinucleotide repeat diseases: for an example and more details see Huntington Disease.[10]
Dominant and recessive genetic diseases in humans
In humans, many genetic traits or diseases are classified simply as "dominant" or "recessive." Especially with respect to so-called recessive diseases, this can oversimplify the underlying molecular basis and lead to misunderstanding of the nature of dominance. For example, the genetic disease phenylketonuria (PKU)[11] results from any of a large number (>60) of alleles at the gene locus for the enzyme phenylalanine hydroxylase (PAH).[12] Many of these alleles produce little or no PAH, as a result of which the substrate phenylalanine and its metabolic byproducts accumulate in the central nervous system and can cause severe intellectual disability if untreated.
The genotypes and phenotypic consequences of interactions among three alleles are shown in the following table:[13]
| Genotype | PAH activity | [phe] conc | PKU ? |
|---|---|---|---|
| AA | 100% | 60 uM | No |
| AB | 30% | 120 uM | No |
| CC | 5% | 200 ~ 300 uM | Hyperphenylalaninemia |
| BB | 0.3% | 600 ~ 2400 uM | Yes |
In unaffected persons homozygous for a standard functional allele (AA), PAH activity is standard (100%), and the concentration of phenylalanine in the blood [phe] is about 60 uM. In untreated persons homozygous for one of the PKU alleles (BB), PAH activity is close to zero, [phe] ten to forty times standard, and the individual manifests PKU.
In the AB heterozygote, PAH activity is only 30% (not 50%) of standard, blood [phe] is elevated two-fold, and the person does not manifest PKU. Thus, the A allele is dominant to the B allele with respect to PKU, but the B allele is incompletely dominant to the A allele with respect to its molecular effect, determination of PAH activity level (0.3% < 30% << 100%). Finally, the A allele is an incomplete dominant to B with respect to [phe], as 60 uM < 120 uM << 600 uM. Note once more that it is irrelevant to the question of dominance that the recessive allele produces a more extreme [phe] phenotype.
For a third allele C, a CC homozygote produces a very small amount of PAH enzyme, which results in a somewhat elevated level of [phe] in the blood, a condition called hyperphenylalaninemia, which does not result in intellectual disability.
That is, the dominance relationships of any two alleles may vary according to which aspect of the phenotype is under consideration. It is typically more useful to talk about the phenotypic consequences of the allelic interactions involved in any genotype, rather than to try to force them into dominant and recessive categories.
Dominant-negative mutations
Many proteins are normally active in the form of a multimer, an aggregate of multiple copies of the same protein, otherwise known as a homomultimeric protein or homooligomeric protein. In fact, a majority of the 83,000 different enzymes from 9800 different organisms in the BRENDA Enzyme Database[14] represent homooligomers.[15] When the wild-type version of the protein is present along with a mutant version, a mixed multimer can be formed. A mutation that leads to a mutant protein that disrupts the activity of the wild-type protein in the multimer is a dominant-negative mutation.
A dominant-negative mutation may arise in a human somatic cell and provide a proliferative advantage to the mutant cell, leading to its clonal expansion. For instance, a dominant-negative mutation in a gene necessary for the normal process of programmed cell death (Apoptosis) in response to DNA damage can make the cell resistant to apoptosis. This will allow proliferation of the clone even when excessive DNA damage is present. Such dominant-negative mutations occur in the tumor suppressor gene p53.[16][17] The P53 wild-type protein is normally present as a four-protein multimer (oligotetramer). Dominant-negative p53 mutations occur in a number of different types of cancer and pre-cancerous lesions (e.g. brain tumors, breast cancer, oral pre-cancerous lesions and oral cancer).[16]
Dominant-negative mutations also occur in other tumor suppressor genes. For instance two dominant-negative germ line mutations were identified in the Ataxia telangiectasia mutated (ATM) gene which increases susceptibility to breast cancer.[18] Dominant negative mutations of the transcription factor C/EBPα can cause acute myeloid leukemia.[19] Inherited dominant negative mutations can also increase the risk of diseases other than cancer. Dominant-negative mutations in Peroxisome proliferator-activated receptor gamma (PPARγ) are associated with severe insulin resistance, diabetes mellitus and hypertension.[20]
Dominant-negative mutations have also been described in organisms other than humans. In fact, the first study reporting a mutant protein inhibiting the normal function of a wild-type protein in a mixed multimer was with the bacteriophage T4 tail fiber protein GP37.[21] Mutations that produce a truncated protein rather than a full-length mutant protein seem to have the strongest dominant-negative effect in the studies of P53, ATM, C/EBPα, and bacteriophage T4 GP37.
History
The concept of dominance was introduced by Gregor Mendel. Though Mendel, "The Father of Genetics", first used the term in the 1860s, it was not widely known until the early twentieth century. Mendel observed that, for a variety of traits of garden peas having to do with the appearance of seeds, seed pods, and plants, there were two discrete phenotypes, such as round versus wrinkled, yellow versus green seeds, red versus white flowers or tall versus short plants. When bred separately, the plants always produced the same phenotypes, generation after generation. However, when lines with different phenotypes were crossed (interbred), one and only one of the parental phenotypes showed up in the offspring (green, or round, or red, or tall). However, when these hybrid plants were crossed, the offspring plants showed the two original phenotypes, in a characteristic 3:1 ratio, the more common phenotype being that of the parental hybrid plants. Mendel reasoned that each parent in the first cross was a homozygote for different alleles (one parent AA and the other parent aa), that each contributed one allele to the offspring, with the result that all of these hybrids were heterozygotes (Aa), and that one of the two alleles in the hybrid cross dominated expression of the other: A masked a. The final cross between two heterozygotes (Aa X Aa) would produce AA, Aa, and aa offspring in a 1:2:1 genotype ratio with the first two classes showing the "A" phenotype, and the last showing the "a" phenotype, thereby producing the 3:1 phenotype ratio.
Mendel did not use the terms gene, allele, phenotype, genotype, homozygote, and heterozygote, all of which were introduced later. He did introduce the notation of capital and lowercase letters for dominant and recessive alleles, respectively, still in use today.
See also
- Introduction to genetics
- Mendelian inheritance
- Evolution of dominance
- List of Mendelian traits in humans
- Mitochondrial DNA
- Punnet square
- Sex linkage
References
- ↑ "dominance". The Oxford American College Dictionary. Oxford University Press via HighBeam Research. Retrieved 5 May 2012. (subscription required)
- ↑ Ridley, Matt (1999). "Disease". Genome: The Autobiography of a Species in 23 Chapters. Harper Collins. pp. 136–146. ISBN 978-0-06-089408-5.
- ↑ King et al., RC (2006). A Dictionary of Genetics (7th ed.). Oxford University Press. p. 129. ISBN 978-0-19-530761-0. "Dominance [refers] to alleles that fully manifest their phenotype when present in the heterozygous ... state."
- ↑ "Cat Coat Color". Veterinary Genetics Laboratory, University of California. Retrieved 2011-11-02.
- ↑ Imes, D. L.; Geary, L. A.; Grahn, R. A.; Lyons, L. A. (April 2006). "Albinism in the domestic cat (Felis catus) is associated with a tyrosinase (TYR) mutation" (Short Communication). Animal Genetics 37 (2): 175–8. doi:10.1111/j.1365-2052.2005.01409.x. PMC 1464423. PMID 16573534.
- ↑ Schmidt-Küntzel, A.; Eizirik, E.; O'Brien, S. J.; Menotti-Raymond, M. (April 2005). "Tyrosinase and tyrosinase related protein 1 alleles specify domestic cat coat color phenotypes of the albino and brown loci". Journal of Heredity 96 (4): 289–301. doi:10.1093/jhered/esi066. PMID 15858157.
- ↑ Pennington, Sandra (1999). 11th Hour: Introduction to Genetics. Wiley. p. 43. ISBN 978-0-632-04438-2.
- ↑ Carr, Steven M. "Extensions to Mendelian Analysis". Memorial University of Newfoundland.
- ↑ 9.0 9.1 Online 'Mendelian Inheritance in Man' (OMIM) Albinism, oculocutaneous, type IA -203100
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) Huntington disease -143100
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) Hyperphenylalaninemia, non-PKU mild -261600
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) Phenylalanine Hydroxylase; PAH -612349
- ↑ Carr, Steven M. "One Gene, One Enzyme". Memorial University of Newfoundland.
- ↑ Schomburg I, Chang A, Ebeling C, et al. (January 2004). "BRENDA, the enzyme database: updates and major new developments". Nucleic Acids Res. 32 (Database issue): D431–3. doi:10.1093/nar/gkh081. PMC 308815. PMID 14681450.
- ↑ Hashimoto K, Nishi H, Bryant S, Panchenko AR (June 2011). "Caught in self-interaction: evolutionary and functional mechanisms of protein homooligomerization". Phys Biol 8 (3): 035007. doi:10.1088/1478-3975/8/3/035007. PMC 3148176. PMID 21572178.
- ↑ 16.0 16.1 Marutani M, Tonoki H, Tada M, et al. (October 1999). "Dominant-negative mutations of the tumor suppressor p53 relating to early onset of glioblastoma multiforme". Cancer Res. 59 (19): 4765–9. PMID 10519380.
- ↑ Goh AM, Coffill CR, Lane DP (January 2011). "The role of mutant p53 in human cancer". J. Pathol. 223 (2): 116–26. doi:10.1002/path.2784. PMID 21125670.
- ↑ Chenevix-Trench G, Spurdle AB, Gatei M, et al. (February 2002). "Dominant negative ATM mutations in breast cancer families". J. Natl. Cancer Inst. 94 (3): 205–15. doi:10.1093/jnci/94.3.205. PMID 11830610.
- ↑ Pabst T, Mueller BU, Zhang P, et al. (March 2001). "Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia". Nat. Genet. 27 (3): 263–70. doi:10.1038/85820. PMID 11242107.
- ↑ Barroso I, Gurnell M, Crowley VE, et al. (1999). "Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension". Nature 402 (6764): 880–3. doi:10.1038/47254. PMID 10622252.
- ↑ Bernstein H, Fisher KM (March 1968). "Dominance in bacteriophage T4D". Genetics 58 (3): 307–18. PMC 1211863. PMID 5662621.
- "On-line notes for Biology 2250 – Principles of Genetics". Memorial University of Newfoundland.
- Online 'Mendelian Inheritance in Man' (OMIM) Hemoglobin—Beta Locus; HBB -141900 — Sickle-Cell Anemia
- Online 'Mendelian Inheritance in Man' (OMIM) ABO Glycosyltransferase -110300 — ABO blood groups
External links
- "Online Mendelian Inheritance in Man" (OMIM)
- "Autosomal dominance of Huntington's Disease". Huntington's Disease Outreach Project for Education at Stanford
- "Examples of incomplete dominance" (YouTube)