Arabinose
| Arabinose | |
|---|---|
 | |
| IUPAC name Arabinose | |
| Other names Pectinose | |
| Identifiers | |
| CAS number | 147-81-9 |
| PubChem | 5460291 |
| ChemSpider | 59687 |
| EC-number | 205-699-8 |
| ChEBI | CHEBI:46983 |
| Jmol-3D images | {{#if:O=C[C@@H](O)[C@H](O)[C@H](O)COC([C@H]([C@H]([C@@H](C=O)O)O)O)O|Image 1 Image 2 |
| |
| |
| Properties[1] | |
| Molecular formula | C5H10O5 |
| Molar mass | 150.13 g mol−1 |
| Appearance | Colorless crystals as prisms or needles |
| Density | 1.585 g/cm3 (20 ºC) |
| Melting point | 164 to 165 °C; 327 to 329 °F; 437 to 438 K |
| Solubility in water | Soluble |
| Hazards | |
| NFPA 704 |
 1
1
0
|
| Related compounds | |
| Related aldopentoses | Ribose Xylose Lyxose |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Arabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde (CHO) functional group.
For biosynthetic reasons, most saccharides are almost always more abundant in nature as the "D"-form, or structurally analogous to D-glyceraldehyde.[note 1] However, L-arabinose is in fact more common than D-arabinose in nature and is found in nature as a component of biopolymers such as hemicellulose and pectin. The L-arabinose operon is a very important operon in molecular biology and bioengineering.
A classic method for the organic synthesis of arabinose from glucose is the Wohl degradation.[2]
D-Arabinose 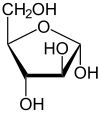
α-D-Arabinofuranose
β-D-Arabinofuranose
α-D-Arabinopyranose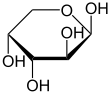
β-D-Arabinopyranose
Etymology
Arabinose gets its name from gum arabic, from which it was first isolated.[3]
Use
Arabinose is used as a culture medium for certain bacteria.
Notes
- ↑ For sugars, the D/L nomenclature does not refer to the molecule's optical rotation properties but to its structural analogy to glyceraldehyde.
References
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-110. ISBN 0-8493-0462-8.
- ↑ Braun, Géza (1940), "D-Arabinose", Org. Synth. 20: 14; Coll. Vol. 3: 101
- ↑ Merriam Webster Dictionary
| |||||||||||||||||||||||||||||||||||||