Absorbance
- "Optical density" redirects here. "Optical density" can also refer to index of refraction.[1]
In spectroscopy, the absorbance (also called optical density[2][3]) of a material is a logarithmic ratio of the radiation falling upon a material, to the radiation transmitted through a material.[4][5] Absorbance measurements are often carried out in analytical chemistry.
In physics, the term spectral absorbance is used interchangeably with spectral absorptance or absorptivity. In this case it has a slightly different meaning: the fraction of radiation absorbed at specific wavelengths.
Detailed explanation
Absorbance is a quantitative measure expressed as a logarithmic ratio between the radiation falling upon a material and the radiation transmitted through a material.
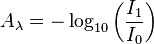 ,
,
where 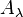 is the absorbance at a certain wavelength of light (
is the absorbance at a certain wavelength of light (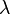 ),
),  is the intensity of the radiation (light) that has passed through the material (transmitted radiation), and
is the intensity of the radiation (light) that has passed through the material (transmitted radiation), and  is the intensity of the radiation before it passes through the material (incident radiation).
is the intensity of the radiation before it passes through the material (incident radiation).
Outside the field of analytical chemistry, e.g. when used in biology and the Tunable Diode Laser Absorption Spectroscopy (TDLAS) technique, the absorbance is often defined using the natural logarithm instead of the common logarithm, i.e. as
The term absorption refers to the physical process of absorbing light, while absorbance refers to the mathematical quantity. Also, absorbance does not always measure absorption: if a given sample is, for example, a dispersion, part of the incident light will in fact be scattered by the dispersed particles, and not really absorbed. However, in such cases, it is recommended that the term "attenuance" (formerly called "extinction") be used, which accounts for losses due to scattering and luminescence.[6]
See the Beer-Lambert law for a more complete discussion.
Although absorbance is properly unitless, it is sometimes reported in "Absorbance Units" or AU. However, many people, including scientific researchers, wrongly state the results from absorbance measurement experiments in terms of arbitrary units.[7]
Logarithmic vs. directly proportional measurements
The amount of light transmitted through a material diminishes exponentially as it travels through the material. Since the absorbance of a sample is measured as a logarithm, it is directly proportional to the thickness of the sample and to the concentration of the absorbing material in the sample. Some other measures related to absorption, such as transmittance, are measured as a simple ratio so they vary exponentially with thickness and concentration of the material.
| Absorbance | Transmittance ( ) ) |
|---|---|
| 0 | 1 |
| 0.1 | 0.79 |
| 0.25 | 0.56 |
| 0.5 | 0.32 |
| 0.75 | 0.18 |
| 0.9 | 0.13 |
| 1 | 0.1 |
| 2 | 0.01 |
| 3 | 0.001 |
Instrument measurement range
Any real measuring instrument has a limited range over which it can accurately measure absorbance. An instrument must be calibrated and checked against known standards if the readings are to be trusted. Many instruments will become non-linear (fail to follow the Beer-Lambert law) starting at approximately 2 AU (~1% Transmission). It is also difficult to accurately measure very small absorbance values (below 10−4) with commercially available instruments for chemical analysis. In such cases, laser-based absorption techniques can be used, since they have demonstrated detection limits that supersede those obtained by conventional non-laser-based instruments by many orders of magnitude (detections have been demonstrated all the way down to 5 10−13). The theoretical best accuracy for most commercially available non-laser-based instruments is in the range near 1 AU. The path length or concentration should then, when possible, be adjusted to achieve readings near this range.
Shade number
Some filters, notably welding glass, are rated by shade number, which is 7/3 times the absorbance plus one:[8]
- shade number

or
- shade number
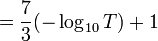
So, if the filter has 0.1% transmittance (0.001 transmittance, which is 3 absorbance units) the shade number would be 8.
Similar terms
Many similar terms are used to describe concepts relating to absorbance and some terms may have differing interpretation or usage in different disciplines.
Absorptance
Absorptance refers to a directly proportional ratio. Absorptance is the ratio of the radiation absorbed by a surface to that incident upon it. Total absorptance refers to absorptance measured over all wavelengths. Spectral absorptance refers to absorptance measured at a specified wavelengths.[9]
Absorptance is a simple ratio, whereas absorbance is a logarithmic ratio. This difference means that the two different measures are often used for different applications. Color and Vision Research Laboratories, Institute of Ophthalmology, UCL, explains:
Absorbance spectra are typically used to define photopigment spectra because their shape, when normalized (i.e., plotted as a fraction of the maximum absorbance), is independent of pigment optical density (pigment concentration). In contrast, the absorptance spectra, like the spectral sensitivity of the human subject, broadens as the optical density increases.[10]
Absorption factor
Same as Absorptance.
Absorptivity
In physics, the term spectral absorbance is used interchangeably with absorptivity, meaning the fraction of radiation absorbed at a given wavelength. In chemistry, absorptivity usually refers to Molar absorptivity, which is the constant  used in the Beer-Lambert law,
used in the Beer-Lambert law,  , where
, where 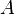 is the absorbance,
is the absorbance,  is the concentration of the solution, and
is the concentration of the solution, and  is the path length.[11]
is the path length.[11]
Mnemonic
A mnemonic to remember the difference between absorbance and absorptance is that absorbance has no t, or not t, meaning it measures all that is not transmitted.
See also
- Optical depth
- Beer-Lambert law
- Transmittance
- Reflectivity
- Tunable Diode Laser Absorption Spectroscopy (TDLAS)
- Densitometry
- Neutral density filter
- Mathematical descriptions of opacity
References
- ↑ Zitzewitz, Paul W. (1999). Glencoe physics. New York, N.Y.: Glencoe/McGraw-Hill. p. 395. ISBN 0-02-825473-2.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "optical density".
- ↑ Laser Guidebook, by Jeff Hecht, p79
- ↑ Mehta, A. UV-Visible Spectroscopy- Derivation of Beer-Lambert Law
- ↑ "Dictionary — Definition of absorptance". Websters-online-dictionary.org. Retrieved 2011-11-21.
- ↑ International Union of Pure and Applied Chemistry (IUPAC) Glossary of terms used in photochemistry. Recommendations 1988 (Braslavsky, S. E. & Houk, K. N., eds) Pure Appl. Chem. 60, 1055-1106 (1988). An updated version, y J. W. Verhoeven, has appeared in Pure Appl. Chem. 68, 2223-2286 (1996).
- ↑ "How to Make Your Next Paper Scientifically Effective — J. Phys. Chem. Lett. 2013, 4, 1578−1581".
- ↑ "How Many? A Dictionary of Units of Measurement by Russ Rowlett". Unc.edu. 2004-09-01. Retrieved 2010-09-20.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "absorbance".
- ↑ http://www-cvrl.ucsd.edu/database/text/intros/intropig.htm
- ↑ "Dictionary — Definition of absorptance". Websters-online-dictionary.org. Retrieved 2011-11-21.
